 |
 |
| Tuberc Respir Dis > Volume 74(1); 2013 > Article |
|
Abstract
Background
Fractional exhaled nitric oxide (FeNO) can be measured easily, rapidly, and noninvasively for the assessment of airway inflammation, particularly mediated by eosinophil, such as asthma. In bronchiectasis (BE), the pathogenesis has been known as chronic airway inflammation and infection with abnormal airway dilatation; however, there are little studies to evaluate the role of FeNO in BE.
Methods
From March 2010 to February 2012, 47 patients with BE, diagnosed by high resolution computed tomography (HRCT), performed FeNO, compared with asthma and chronic obstructive pulmonary disease (COPD). All patients carried out a complete blood count including eosinophil count, chemistry, sputum examination, and spirometry, if indicated. A retrospective analysis was performed to elucidate the clinical role of FeNO in BE patients.
Results
The mean FeNO levels in patients with BE was 18.8±1.5 part per billion (ppb), compared to 48.0±6.4 and 31.0±4.3 in those with asthma and COPD, respectively (p<0.001). The FeNO levels tended to increase along with the disease severity scores by HRCT; however, it was statistically not significant. FeNO in BE with a co-infection of nontuberculous mycobacteria was the lowest at 17.0±3.5 ppb among the study population.
Nitric oxide (NO) is an important inflammatory mediator of biological function in the airways, regulating airway smooth muscle contractility, mucus secretion and vasodilation1 and its measurement in the exhaled air is one of the useful markers for airway inflammation2. It is well-known that NO in the exhaled air of asthmatic patients has been elevated; additionally, it can be also detected in various pulmonary diseases3, such as chronic obstructive pulmonary disease (COPD), cystic fibrosis, pulmonary hypertension, bronchiectasis (BE)4, and even pulmonary tuberculosis (TB)5. The fraction of exhaled NO (FeNO) can be measured simply, noninvasively, highly reproducibly, and has been well established in research and numerous studies have provided evidence regarding the applications of exhaled NO measurements in clinical practice6. However, there are not enough literatures to provide specific guidelines for its application to other pulmonary conditions except eosinophilic airway disease, such as asthma.
In BE, which has been characterized by irreversible airway dilatation due to a structural abnormality of the bronchial wall that leads to delayed mucociliary clearance and more susceptibility to bacterial infection7, FeNO has been also tried as early recognition of diagnosis, assessment of its severity, and response to treatment8. Still not fully understood the pathogenesis of BE, it is definitely true that chronic inflammatory process in the walls of affected airways, with activation of mononuclear cells can also produce large amount of NO. However, conflicting results has been yielded among studies regarding to usefulness to assess airway inflammation by measuring FeNO in BE. In 1995, Kharitonov et al.4 showed that elevated levels of exhaled NO in BE, as in asthma, which was correlated with disease severity. In contrast, separate studies by Tsang et al.9, Ho et al.10, and Narang et al.11 had found no significant difference in FeNO in BE, compared to healthy controls. Recently, only one small study12 revealed that elevated peripheral airway NO, which was calculated by using linear equation of two compartment model of pulmonary exchange dynamics, relates to disease severity measured by lung function and high resolution computed tomography (HRCT) scan and correlates with the quality of life score, however, this study is descriptive and observational study, not designed to compare to other chronic inflammatory airway diseases.
In addition, the measurement of FeNO would have a potential role of detecting TB patients in several papers5,13, and in Tsang et al.'s study9 as mentioned above, FeNO appears to be reduced among BE patients with Pseudomonas aeruginosa infection independent of other clinical parameters. This means that the inflammatory response of activated inflammatory cells, such as mononuclear cells or neutrophils, to different microbes in airways maybe possible assessed by the measurement of FeNO. In Korea, there is a rapid increase in the prevalence of nontuberculous mycobacterial (NTM) infections14, especially in patients who have diagnosed with BE, however, there is also no study to measure FeNO levels in patients with NTM infection.
We have, therefore, conducted this study to measure FeNO in patients with stable BE, compare its levels to alleged chronic inflammatory airway disease, including asthma and COPD and determine its reflection of disease severity assessed by HRCT and potential role of detecting NTM infections combined with BE.
Patients with chronic stable, suspicious BE who visited the outpatient clinic of respiratory center at Seoul National University Bundang Hospital were prospectively enrolled from March 2010 to February 2012. For control groups, patients with chronic cough due to asthma, COPD and other respiratory diseases were also enrolled during same periods, matched with age and sex. Subjects who could not be diagnosed finally or could have mixed diseases, such as COPD and BE were initially excluded. As routine primary clinician's decision, white blood cells with differential count (including eosinophil count), inflammatory markers such as erythrocyte sedimentation rate and C-reactive protein, total IgE level (if indicated), simple chest radiography with or without paranasal sinus view and spirometry with bronchodilator response test were done within a week after first visit. All patients with suspicious BE were underwent CT scan for confirmation of the diagnosis. In Korea, as cystic fibrosis is a kind of rare disease, we postulated that all BE patients were not associated with cystic fibrosis. Ordinary bacterial respiratory Gram's stain and culture, acid-fast bacilli smear and culture were also performed in patients with BE. This study was reviewed by the institutional review board of our hospital and informed consents were exempt because of no intervention trials and retrospective study by electronic medical record review.
In accordance with the American Thoracic Society (ATS) guideline6, FeNO levels were measured as followings: the study subjects were asked to inhale a maximum amount of air outside the valve and exhale into the valve connected to the chemiluminescence NO analyzer, the NIOX MINO (Aerocrine AB, Solna, Sweden). We used the mean value of FeNO levels that were performed two times for each patient, who were followed by clinician's schedule.
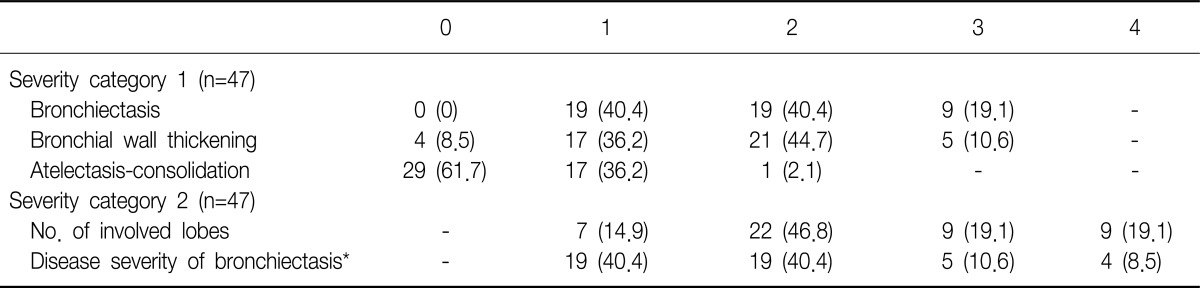
All HRCT scans in patients with BE were scored using "simplified" HRCT scoring systems. Oikonomou et al.15 suggested this semi-quantitative scoring system by using three common parameters, "severity of BE," "severity of bronchial wall thickening," and "atelectasis-consolidation," in which each score ranged from 0 (no abnormalities) to 3 (maximal abnormalities). Details of developing scoring system are showed in Oikonomou et al.'s study15. Then, we also counted the numbers of lobes, in which any abnormalities were shown from right upper lobe to left lower lobe. The final disease severity of BE was calculated as total summation of three common parameters' scores divided by the number of total involved lobes.
Results are expressed as mean values±standard deviation or case numbers/total patients (%). A student's t-test or ANOVA was used to evaluate differences in the level of FeNO between the BE and non-BE groups. The relationship among lung function by spirometry, eosinophil count calculated from total white blood cells (WBCs) and their differential count and FeNO were analyzed using Pearson correlation coefficients. Statistical analyses were performed using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA). The p<0.05 indicated statistical significance.
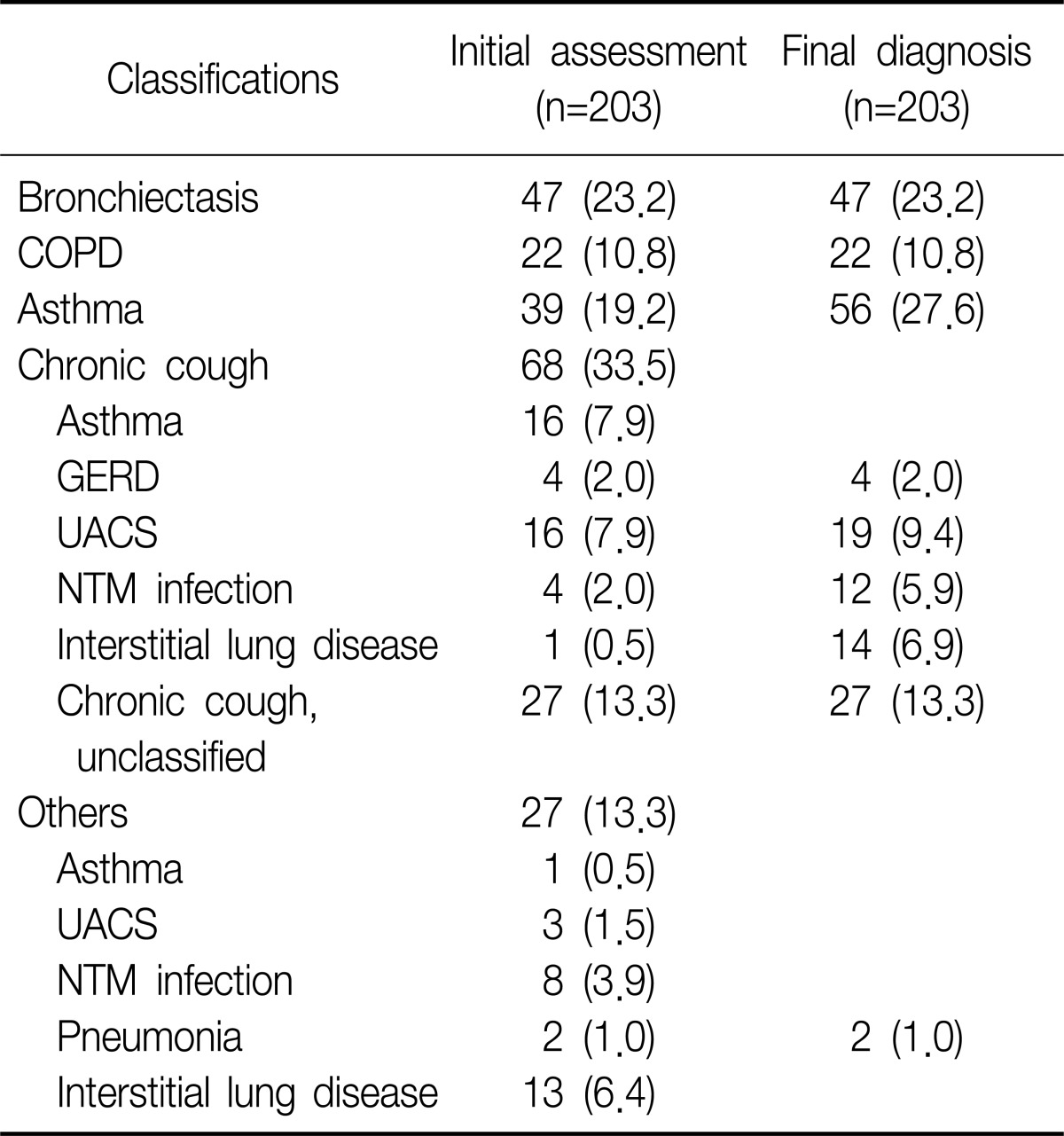
Two hundred three patients were initially enrolled in this study. Of all patients, initial diagnosis was BE in 47 patients, asthma in 39, and COPD in 22. Of remained 95 patients, 68 patients visited outpatient clinic with chronic cough as initial chief complaint were finally diagnosed as various respiratory diseases, such as upper airway cough syndrome (UACS), gastroesophageal reflux disease (GERD), pneumonia, interstitial lung disease (ILD), and NTM infection. The last 27 patients were also categorized as same as chronic cough group and the number of cases that matched with same diagnosis were added in chronic cough group. In final analysis, we excluded 66 patients, whose diagnosis were non-airway, non-respiratory diseases (UACS, GERD, ILD, and pneumonia) or unclassified chronic cough, from 95, then additional 17 patients were diagnosed by asthma. Twelve patients with NTM infections were only enrolled in cases of comparison NTM vs. non-NTM infections (Table 1).
Among the 47 patients with BE, female were 29 (61.7%) of all patients and the median age was 63 years. Current smoker were only 6 (12.8%), most common symptoms sputum (95.7%), following cough (34.0%), dyspnea (27.7%). Mean forced expiratory volume in 1 second (FEV1) was 2.0 L/min, total WBCs and eosinophil count 7,000/mm3 and 181/mm3. The proportion of co-infection with NTM was 25.5%, and there were no patients with co-infection of NTM in asthma and COPD group. Other details in asthma and COPD patients were showed in Table 2.
Overall, the association between FeNO levels and eosinophil count was moderate strong positive with correlation coefficients 0.429 (p<0.001), and same positive but slightly weak between FeNO levels and FEV1 with 0.229 (p<0.05) (Figure 1). However, in BE group, these correlations were not seen in both cases (data were not showed).
FeNO levels of BE group regardless of NTM co-infection were 18.8±1.5 part per billion (ppb), significantly lower than those of chronic inflammatory airway disease, especially asthma group (48.0±6.4 ppb, p<0.001) (Figure 2). All subjects in BE group had FeNO levels of <50 ppb, except for one; this patient had FeNO level of 54 ppb, and 39 (83.0%) of total 47 in BE group had FeNO levels of ≤25 ppb. FeNO levels were showed a decreasing trend from asthma to BE, especially the lowest levels of FeNO was observed in patient BE who had co-infection with NTM, which was 6 ppb. In 60 patients who presented chronic cough with stable condition as initial chief complaint, this trend was same with statistical significance (p=0.043, data not shown).
There were 15 patients in BE group who had two and more than FeNO levels as follow-up with median 5.9 months (range, 0.5-7.7 months), in which there were no significant interval changes between FeNO levels except two patients. One had the highest FeNO levels of 54 ppb among all of BE patients, followed the levels for seven times during one year; the other had the second highest levels of 45 ppb, for three times during four months.
The disease severity assessed by simplified HRCT scores was showed in Table 3. Adjusted by numbers of involved lobes, final severity category was assigned from 1 (minimal abnormalities) to 4 (maximal abnormalities). It was not statistically significant, there was an increasing trend in mean FeNO levels categorized by disease severity of BE (p=0.542) (Figure 3). Coincidently, patients with NTM infection had significant lower FeNO levels than without NTM infection whether combined with BE or not (18.9±2.1 ppb vs. 35.8±3.5 ppb, p<0.05; data not shown in figure). In BE group, there was no difference of FeNO levels whether NTM co-infection or not (19.5±1.6 ppb vs. 17.0±3.5 ppb, p=0.990) (Figure 2).
This study showed that FeNO levels in the patients with BE were significantly lower than those in other chronic inflammatory airway diseases, such as asthma and COPD. Although being statistically insignificant, the trends of FeNO levels increased according to disease severity assessed by simplified HRCT scoring system. Coincidentally, in patient BE with NTM infection, FeNO levels were the lowest among study population, to the best our knowledge, this is the first report to analyze FeNO levels in patients with BE and/or NTM co-infection.
Recently, the clinical application of FeNO has been investigated extensively as a complementary tool to other ways of assessing airway disease3, especially including asthma. The official ATS clinical practice guideline summarized advances in technology and standardization have made FeNO measurement simple, permitting its use as a biomarker that adds a new dimension to the traditional clinical tools in the assessment and management of airways diseases. FeNO offers added advantages for patient care including to detecting eosinophilic airway inflammation, determining and monitoring the responsiveness of corticosteroid, and additionally applying to other situations such as COPD, pulmonary hypertension, and cystic fibrosis6. In our study, FeNO levels had marked lower in patients with BE, especially co-infection with NTM. Currently, there are no definite guidelines of "normal" values of FeNO, suggesting the use of cut points rather than reference values when interpreting FeNO levels in clinical settings. However, this recommendation is used to be only applicable to eosinophilic airway diseases, such as asthma, and still controversial in various inflammatory airways diseases. We thought these results could be a good evidence of applying FeNO measurement to non-eosinophilic inflammatory airway diseases, such as BE and it might be a possible screening method to differentiate BE with other chronic airway diseases at early, clinically stable state.
Interestingly, lower levels of FeNO in patients with NTM infection was unexpected result of this study. In fact, there are a few studies to evaluate the role of FeNO in pulmonary TB. Wang et al.5 elucidated that the increase in exhaled NO observed in patients with active pulmonary TB and explained it was due to an up-regulation of inhaled NO synthase expression in alveolar macrophages which have an enhanced capacity for NO production. Van Beek et al.13 tried to reveal the possibility of measuring exhaled NO as inexpensive screening tools for active TB and Idh et al.16 showed that in both human immunodeficiency virus (HIV) negative and HIV co-infected TB patients, low levels of exhaled NO compared to blood donors and household were observed. However, all of these studies have included only Mycobacterium tuberculosis, to our knowledge, this is the first report to suggest the possible role of FeNO in NTM infections and it is cautiously considerable to use the measurement of FeNO as new, noninvasive screening technique that make a differentiate NTM infections. However, the microbiological identifications of NTM infections were not carried out, which could have revealed the relationship of FeNO and specific pathogens.
We used "simplified" HRCT scoring system to assess the disease severity of BE patients, it has been well-established in many studies. However, in clinical settings, it seems that the radiographic severity would not be always main surrogate marker of actual or functional disease status of BE17. The reproducibility of CT is poor because of radiation risk, cost-effectiveness, and the correlation with symptoms or pulmonary function tests is not well verified. In our study, FeNO levels in BE patients had showed a tendency toward increasing direction as disease severity assessed by HRCT scoring system, even though overall levels of FeNO in BE group were lower than 25 ppb, which was the lowest cut-off values by recent guideline6. This findings means that non-eosinophilic inflammatory process that produce NO could be related to pathogenesis of BE. The overall lower levels of FeNO might be explained by the structural damages of lung parenchyma in most patients with BE compared to asthma, which has little anatomical loss of airway and parenchyma.
There were several limitations in this study. First, these results were elucidated from retrospective analysis and we could not thoroughly deal with other variables that effect NO productions, such as smoking history. Similarly, as the heterogeneity among each clinical diagnostic group inevitably existed such as in symptoms, eosinophil count, FEV1, or inflammatory markers, the correlations of FeNO and eosinophil count or FEV1 could also have been considered as a result of ecological error, a kind of statistical issues related to retrospective analysis. In addition, it can be possible a few patient had mixed diagnosis, for example, BE combined asthma. In fact, one patient in BE group as mentioned above had highly elevated FeNO (54 ppb), treated with systemic steroid and consequentially FeNO levels decreased after treatment. The patient's CT findings also showed definitely severe bronchiectatic changes and no evidences of airway hyper-responsiveness were observed in dynamic pulmonary function test. If we excluded this patient in BE group, our data would have showed more significant results. Finally, our study did not head to evaluate the role of monitoring treatment, we could not follow-up serial FeNO levels regularly, simply focused the initial FeNO levels. Actually, there was one study reporting the relationship of FeNO and exacerbation of BE were focused12, however, all of our study population were in stable conditions because this study was conducted by outpatient-based setting.
In conclusions, FeNO levels in stable BE was lower than other inflammatory airway diseases, especially compared to asthma and the lowest in BE group with co-infection of NTM. It might be also used as a simple, reproducible and noninvasive tool of evaluating disease severity of BE, however, further large-scaled, prospective studies needed to expand the clinical application of measuring FeNO to BE and its subgroup populations.
References
1. Nathan C, Xie QW. Regulation of biosynthesis of nitric oxide. J Biol Chem 1994;269:13725-13728. PMID: 7514592.


2. Kim SH, Yoon HJ. Use of the exhaled nitric oxide for management of asthma and respiratory diseases. Korean J Med 2008;74:579-586.

3. Barnes PJ, Dweik RA, Gelb AF, Gibson PG, George SC, Grasemann H, et al. Exhaled nitric oxide in pulmonary diseases: a comprehensive review. Chest 2010;138:682-692. PMID: 20822990.


4. Kharitonov SA, Wells AU, O'Connor BJ, Cole PJ, Hansell DM, Logan-Sinclair RB, et al. Elevated levels of exhaled nitric oxide in bronchiectasis. Am J Respir Crit Care Med 1995;151:1889-1893. PMID: 7767536.


5. Wang CH, Liu CY, Lin HC, Yu CT, Chung KF, Kuo HP. Increased exhaled nitric oxide in active pulmonary tuberculosis due to inducible NO synthase upregulation in alveolar macrophages. Eur Respir J 1998;11:809-815. PMID: 9623681.


6. Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med 2011;184:602-615. PMID: 21885636.



9. Tsang KW, Leung R, Fung PC, Chan SL, Tipoe GL, Ooi GC, et al. Exhaled and sputum nitric oxide in bronchiectasis: correlation with clinical parameters. Chest 2002;121:88-94. PMID: 11796436.


10. Ho LP, Innes JA, Greening AP. Exhaled nitric oxide is not elevated in the inflammatory airways diseases of cystic fibrosis and bronchiectasis. Eur Respir J 1998;12:1290-1294. PMID: 9877479.


11. Narang I, Ersu R, Wilson NM, Bush A. Nitric oxide in chronic airway inflammation in children: diagnostic use and pathophysiological significance. Thorax 2002;57:586-589. PMID: 12096200.



12. Shoemark A, Devaraj A, Meister M, Ozerovitch L, Hansell DM, Wilson R. Elevated peripheral airway nitric oxide in bronchiectasis reflects disease severity. Respir Med 2011;105:885-891. PMID: 21398103.


13. Van Beek SC, Nhung NV, Sy DN, Sterk PJ, Tiemersma EW, Cobelens FG. Measurement of exhaled nitric oxide as a potential screening tool for pulmonary tuberculosis. Int J Tuberc Lung Dis 2011;15:185-192. PMID: 21219679.

14. Ryoo SW, Shin S, Shim MS, Park YS, Lew WJ, Park SN, et al. Spread of nontuberculous mycobacteria from 1993 to 2006 in Koreans. J Clin Lab Anal 2008;22:415-420. PMID: 19021271.



15. Oikonomou A, Tsanakas J, Hatziagorou E, Kirvassilis F, Efremidis S, Prassopoulos P. High resolution computed tomography of the chest in cystic fibrosis (CF): is simplification of scoring systems feasible? Eur Radiol 2008;18:538-547. PMID: 18040691.



16. Idh J, Westman A, Elias D, Moges F, Getachew A, Gelaw A, et al. Nitric oxide production in the exhaled air of patients with pulmonary tuberculosis in relation to HIV co-infection. BMC Infect Dis 2008;8:146PMID: 18950489.




17. Robroeks CM, Roozeboom MH, de Jong PA, Tiddens HA, Jobsis Q, Hendriks HJ, et al. Structural lung changes, lung function, and non-invasive inflammatory markers in cystic fibrosis. Pediatr Allergy Immunol 2010;21:493-500. PMID: 20546526.


Figure 1
Correlation analysis of fractional exhaled nitric oxide (FeNO) and eosinophil count (A), forced expiratory volume in 1 second (FEV1) (B).
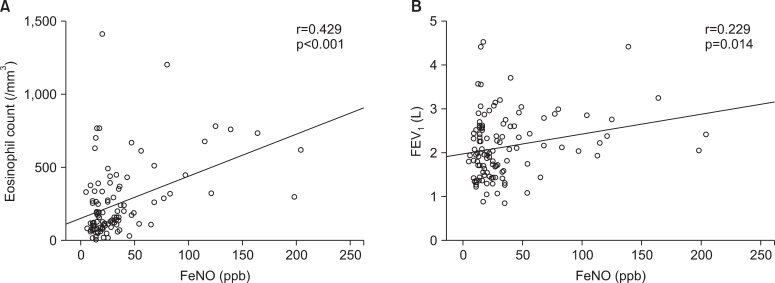
Figure 2
Comparison of fractional exhaled nitric oxide (FeNO) levels in the bronchiectasis (BE) and non-BE patients (n=125, all numeric data in the bottom row were showed by mean values±standard errors). In each group, the bottom and top of the box means the 25th and 75th percentile (the lower and upper quartiles, respectively), and the thick line is the median value. The ends of the whiskers (thin lines) represent the lowest datum still within 1.5 interquartile ranges (IQR) of the lower quartile, and the highest datum still within 1.5 IQR of the upper quartile. Blanked circles mean outliers; 139.0 in asthma, 97.0 and 65.0 in chronic obstructive pulmonary disease (COPD), 54.0 and 40.0 in BE without nontuberculous mycobacteria (NTM) infection (BE-NTM), and 45.0 and 39.0 in BE with NTM infection (BE+NTM). *p<0.001, **p<0.05, ***p=0.990.
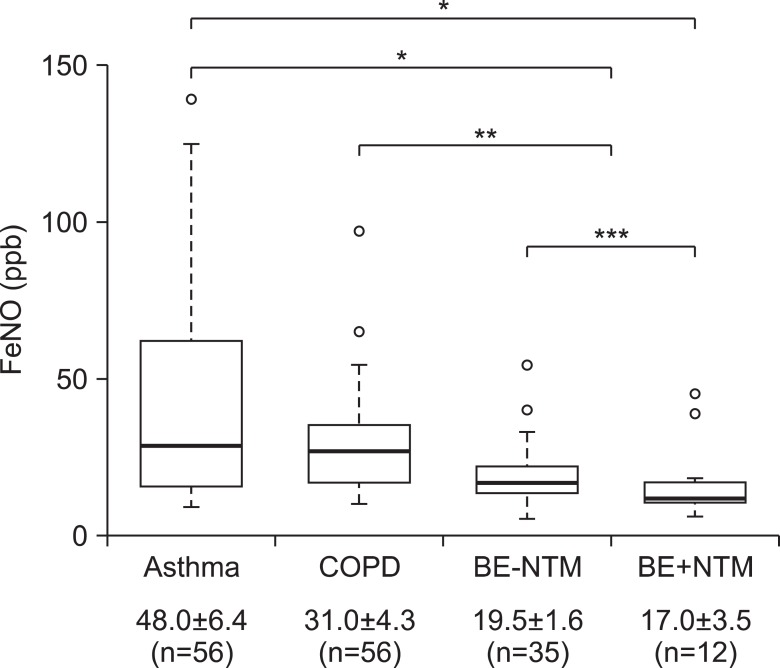
Figure 3
Fractional exhaled nitric oxide (FeNO) and disease severity assessed by high resolution computed tomography (HRCT) in patients with bronchiectasis. Dots represent mean level of FeNO in each group. Dot lines represent the lowest datum still within 1.5 interquartile ranges (IQR) of the lower quartile, and the highest datum still within 1.5 IQR of the upper quartile.
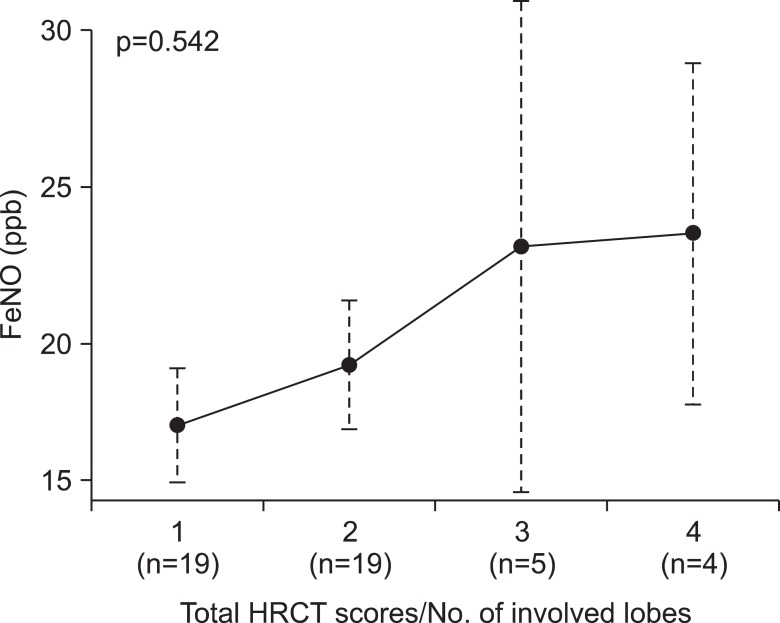
Table 2
Baseline characteristics of study population
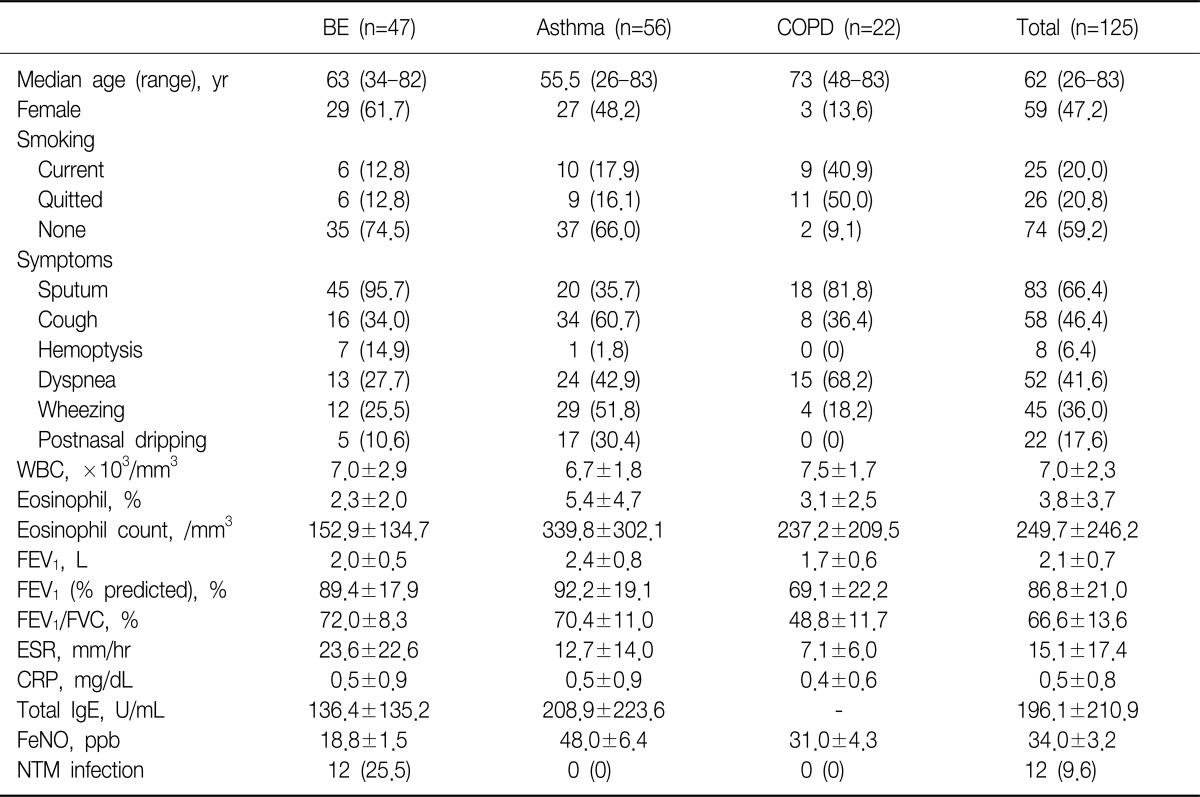
Values are presented as mean±standard deviation or number (%).
BE: bronchiectasis; COPD: chronic obstructive pulmonary disease; WBC: white blood cell; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; ESR: erythrocyte sedimentation rate; CRP: C-reactive protein; FeNO: fractional exhaled nitric oxide; NTM: nontuberculous mycobacteria.
- TOOLS
-
METRICS

- Related articles


 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Print
Print Download Citation
Download Citation



