 |
 |
| Tuberc Respir Dis > Volume 81(3); 2018 > Article |
|
Abstract
Chronic obstructive pulmonary disease (COPD) is a frequent comorbid condition associated with increased morbidity and mortality. Pneumonia is the most common infectious disease condition. The purpose of this review is to evaluate the impact of pneumonia in patients with COPD. We will evaluate the epidemiology and factors associated with pneumonia. We are discussing the clinical characteristics of COPD that may favor the development of infections conditions such as pneumonia. Over the last 10 years, there is an increased evidence that COPD patients treated with inhaled corticosteroids are at increased risk to develp pneumonia. We will review the avaialbe information as well as the possible mechanism for this events. We also discuss the impact of influenza and pneumococcal vaccination in the prevention of pneumonia in COPD patients.
Chronic obstructive pulmonary disease (COPD) is the leading cause of death for both males and females in the United States and is projected to rise in ranking by 20201. According to data from the National Center for Health Statistics of the Centers for Disease Control and Prevention, COPD became the third leading cause of death by 20082. Furthermore, according to the World Health Organization in 2014, lower respiratory tract infections and COPD represented the third and fourth leading causes of death worldwide3. In addition, community acquired pneumonia is cause of morbidity and mortality around the world. Pneumonia is the seventh leading cause of death overall and first leading cause of infectious death in the United States4 and Europe5. Pneumonia was associated with more than 1.1 million inpatient hospitalizations and 50,000 deaths in 20106,7 the vast majority of deaths due to pneumonia occur in patients over 65 years of age. This condition is responsible for a high financial burden with over $10 billion spent caring for patients with pneumonia6,7. Therefore, it is important to understand the association between COPD and pneumonia, as well as their impact in patient's management.
COPD alone affects 20 million Americans, and is one of the most frequently reported comorbid conditions in pneumonia patients8,9,10,11,12. Clinical studies of pneumonia including outpatient, inpatient and intensive care unit (ICU) cohorts have shown that COPD is a frequently reported comorbid condition (Figure 1)13,14,15,16,17. Compared to patients without COPD, pneumonia patients with COPD are likely to have more severe pneumonia, increased number of hospital admissions, and worse outcome18,19,20. In the first year after a COPD diagnosis, individuals are at 16 times the risk for pneumonia compared to those without COPD21. In a recent study the incidence rate of community acquired pneumonia was 22.4 events per 1,000-person years in the 10 years following the diagnosis of COPD, and more than 50% higher in those categorized as having severe COPD22. Furthermore, the economic impact of pneumonia is greater for those with COPD, illustrated by a doubling of direct medical costs following an inpatient hospitalization for pneumonia compared to those without COPD in a study of older individuals. More recent studies evaluated the risk of pneumonia in COPD patients that also have other co-morbid conditions such as cardiovascular disease (CVD). COPD patients with CVD had increased risk of pneumonia23. Lin et al.23 reported that COPD patients with CVD who received inhaled corticosteroids (ICS)-containing therapy had significantly increased risk of developing pneumonia compared to those who did not receive ICS-containing therapy or those who only had comorbid CVD. The increased incidence of pneumonia in COPD patients using ICS is discussed latter.
Despite COPD being one of the most frequent comorbid conditions and a risk factor for developing pneumonia, it has not been recognized as an increased risk factor for mortality in pneumonia patients24,25,26. Furthermore, in the well-validated prediction rule developed as part of the pneumonia Patient Outcomes Research Team (PORT) cohort study, that evaluated 30-day mortality in patients with pneumonia, excluded chronic pulmonary disease as a risk factor27. This prediction rule was based on 20 variables that included five comorbid illnesses (cardiovascular, history of malignancy, cerebrovascular, renal and liver diseases)27. In addition, Fine et al.11 published a meta-analysis related to prognosis and outcomes in community-acquired pneumonia (CAP) patients, and found that patients with pulmonary diseases, including COPD, asthma and interstitial lung disease, did not show higher mortality. However, in previous research (PORT studies and the metaanalysis), the diagnosis of COPD was combined with asthma and interstitial lung diseases, which might be inaccurate given that these conditions exhibit different natural histories, and may bias the overall impact of COPD on pneumonia morbidity and mortality.28 Restrepo et al.19 reported that COPD patients hospitalized with pneumonia, compared to patients without COPD, show significantly higher 30- and 90-day mortality and latter Rello et al.20 showed also increased mortality in pneumonia patients with COPD that required mechanical ventilation. In addition, hospitalized pneumonia patients with COPD exhibited significantly higher rates of ICU admission and a longer length of hospital stay compared with those without COPD. However, a systematic review and meta-analysis of 11 studies (cohort [n=9] and case-control [n=2]) showed that COPD was not associated with increased mortality in cohort studies and reduced mortality in cases-control studies of hospitalized patients with pneumonia29. In addition, COPD was not associated with longer hospital stay and more need for mechanical ventilation. Therefore, despite a higher risk to develop pneumonia the current evidence suggest that COPD may not be associated win increased morbidity and mortality in patients hospitalized with pneumonia. However, some of these studies had important limitations such as an imprecise COPD and pneumonia diagnosis. Furthermore, distinguishing among pneumonic and non-pneumonic exacerabtions in COPD patients is still a matter of controversy in the big epidemiological studies. For all that reasons, prospective population-based cohort studies are needed to further clarify this issue.
The mucosal surface of the COPD patient's lung is constantly exposed to microbial pathogens that have the potential to cause pneumonia in susceptible hosts. The risk of developed pneumonia could be related to host related factors, or microbiome changes that allow increased presence of pathogenic organisms. Microbiome imbalances can contribute to disease as they disrupt normal micro-environmental stimuli for the human host30. An effective early immune response in the lower respiratory tract is crucial for a successful balance of the microbiome. Cells of the innate immune system possess germline-encoded pattern-recognition receptors that can sense conserved microbial molecules referred to as pathogen associated molecular patternsand set off a cascade of immune responses. Among pattern-recognition receptors, nucleotide-binding and oligomerization domain-like receptors are unique cytosolic receptors, which constantly patrol for changes in pathogens in cytoplasm. There is intense research to describe inflammasome assembly, activation, and their role in acute pneumonia31. Furthermore, understanding the interactions between different inflammasomes during the innate immune response is essential for identifying how immune sensors are stimulated by ligands and ultimately, for development of therapies to attenuate excessive tissue damage.
COPD patients may be more susceptible to develop pneumonia based on their clinical characteristics such as having chronic bronchitis with persistent mucus production, and the presence of potential pathogenic bacteria in the airways, the presence of bacteria in the airway in stable COPD patients and increased numbers during exacerbations have been associated with increased inflammation and the host immune response32. Chronic bronchitis in COPD is seen more frequent in persistent smokers and has been associated with increased disease progression, and more frequent exacerbations32. This is likely since chronic bronchitis is associated with airway infection. Mucus production is an important feature in COPD patients with chronic bronchitis. Mucus that is formed in the airways is a protective barrier composed of water, salt and proteins. The major macromolecular components of the mucus are proteins called mucins33. Experimental studies have demonstrated that mucin secretion is required for defense against bacterial infections, linking mucin deficiency with chronic airway infections. Airway mucins have been shown to be an important airway mucus transport, leading to sputum production, increased airway inflammation, infection, worsening airflow obstruction and markers of disease progression34. In moderate COPD, increases of MUC5AC and MUC5B have been detected compared to non-smokers and smokers without airway obstruction35, although these findings have not been related to airway infection. In non-cystic fibrosis (CF) bronchiectasis, elevated MUC2 levels were related to the presence of Pseudomonas aeruginosa and disease severity36. Recent Sibila et al.37 reported that airway MUC2 levels are decreased in severe COPD patients colonized by positive pathogen microorganism. These studies suggest that mucins changes may be one of the mechanisms underlying airway bacterial changes in COPD patients, and may be associated with presence of pathogenic bacteria; but its role in the development of pneumonia has not been described.
Braeken et al.38 reported the associations between COPD and pneumonia in a large population-based study. The authors discussed potential smoking-induced mechanisms leading to increased risk of pneumonia in COPD, such as host physiological and structural changes, increased bacterial virulence and impaired host immunity. Shukla et al.39,40 found increased respiratory tract epithelial expression of specific bacterial adhesion factors in COPD, platelet-activating factor receptor (PAFr) which is the major pneumococcal and Haemophilus influenzae adhesion molecule. The authors suggested that this could be one important mechanism that could significantly increase the risk of Streptococcus pneumoniae respiratory infection in COPD. Pack-years of smoking were strongly related to epithelial PAFr protein levels in COPD patients40. Furthermore, the authors also found that S. pneumoniae expresses phosphorylcholine in its cell wall that specifically binds to PAFr, leading to initial attachment and subsequent translocation of bacteria into deeper tissue. Translational research in this area of bacterial-epithelial interactions can provide novel insights into pathogenesis of pneumonia in COPD patients, its natural history, as well as new therapeutic targets. Blocking the initial stages of bacterial adhesion and colonization in already activated epithelium in COPD patients could emerge as a promising target for the development of alternate, non-antibiotic pharmacotherapies for the management of the disease and its infective complication41. Therefore, there are multiple factors in COPD patients that may predispose them to have an increased risk factor for development of pneumonia (Table 1).
Understanding of the role of bacteria in patients with stable COPD, and how potentially pathogenic microorganisms isolated in these patients under stable conditions can contribute to pneumonia is not well known. Some studies suggested that these bacteria contribute to chronic airway inflammation leading to COPD progression and increased risk to develop pneumonia42,43. More important, since the description of the lung microbiome on healthy individual using molecular culture-independent techniques have identified that normal airway has multiple bacteria species and these are different in patients with underlying lung conditions like COPD. Analysis of the highly conserved 16S rRNA gene has been used to assign phylogeny and allowed picture of the complete microbial community in in the respiratory tract including upper airway, sinus, and bronchial tree44. The number of studies examining the lower airways microbiome have significant increased over the past few years and they describe the differences in bacteria philia in patient with chronic disease including COPD, asthma and healthy individuals30,45. A study reported a significantly different bacterial community in patients with very severe COPD compared with nonsmokers, and among smokers compared to patients with CF46. Clinical studies are needed to understand the role of bacteria microbiomes in COPD patients and the risk of pneumonia. Furthermore, we need to understand the impact of antibiotics, given for either acute exacerbations, or chronic long-term administration, on these bacterial communities and pneumonia.
Liapikou et al.47 reported in a study of severe pneumonia patients with COPD that microbiological diagnosis occurred in 46% patients, and blood cultures were diagnostic in 12% of cases. The most frequent microorganism identified in COPD patients with pneumonia was S. pneumoniae . Other investigators also reported that in elderly patients with COPD and pneumonia, S. pneumoniae was the most frequent organisms isolated48. Patients with COPD also had more infections attributable to P. aeruginosa, but fewer attributable to Legionella pneumophila compared to non-COPD patients, respectively. Other studies suggest that hospitalized pneumonia patients with COPD have more infections attributable to P. aeruginosa, particularly in those patients with bronchiectasis19,49. Other risk factors for Pseudomonas and other potentially drugresistant pathogen such as previous isolation, ICU admission, immunosupresion and prior antimicrobial therapy (<90 days) have been described in COPD patients50. These data support the Infectious Diseases Society of America and American Thoracic Society (IDSA/ATS) recommendation that appropriate diagnostic procedures and anti-pseudomonas coverage should be considered in pneumonia patients with severe COPD, whether bronchiectasis is present, particularly those treated with corticosteroids51. Therefore, it is important to recognize COPD in patients with pneumonia so that they may receive appropriate antimicrobial therapy.
ICS are anti-inflammatory agents widely used in respiratory medicine. Their established efficacy and safety profile have placed this class of medications at the current treatment recommendations in chronic respiratory diseases such as asthma and COPD52,53. In COPD ICS have demonstrated to reduce the overall frequency of exacerbations and improve quality of life53,54,55. Paradoxically, several large trials have demonstrated that the use of ICS was associated with an increased incidence of pneumonia in COPD patients22,56,57,58,59,60,61,62,63 (Table 2). Festic and Scanlon64 reported systematic literature review identified randomized controlled trials (RCTs) that had pneumonia measured as a safety or adverse effect; these trials reported an increased risk of pneumonia. The most studied medication was fluticasone, followed by budesonide and mometasone. TORCH was the largest RCT; it included more than 6,000 patients and was the first trial to show significantly increased risk of pneumonia (hazard ratio, 1.64; 95% confidence interval [CI], 1.33-2.02)56. The risk of developing pneumonia increased with duration of therapy, dose, age and disease severity. Several other trials demonstrated increased risk of pneumonia among ICS users22,57,58,59,61,62,63. This report64 also reported the risk of pneumonia in COPD patients using ICS from observational studies65,66,67,68. All observational studies showed increased risk of pneumonia. Several of the RCTs of ICS in COPD have reported unadjusted risk of pneumonia-related mortality; none found a difference between ICS and non-ICS arms22,56,57,58,59,60,61,62,63,64. Several observational studies reported either similar or lesser mortality among ICS users, despite increased risk of pneumonia65,66,67. A study of Veterans Affairs (VA) hospitals assessed the association of ICS exposure with mortality for hospitalized subjects with pneumonia that had COPD65,66. The use of ICS showed a protective effect with an unadjusted relative risk of 0.50 (95% CI, 0.41-0.60) for 30-day mortality. Joo et al.67 analyzed a dataset from the VA and Centers for Medicare and Medicaid Services, also showed decreased risk of 30-day mortality followed admission for pneumonia. Some of these studies also reported an improvement in other pertinent outcomes among patients using ICS, such as decreased risk of parapneumonic effusion and less frequent need for mechanical ventilation and use of vasopressors65,66,68,69,70,71.
Some studies have related ICS use with potentially drug resistant pathogens. Sibila et al.72 showed COPD patients hospitalized with pneumonia that prior outpatient use of ICS was associated with a higher severity of illness at admission and antimicrobial drug-resistant pathogens. This study found that ICS was not associated with higher mortality and/or length of hospitalization. Liapikou et al.47 reported that COPD patients treated with chronic ICS had a higher rate of pneumonia due to P. aeruginosa but less Legionella spp. infection. However, antimicrobial resistance was not assessed in COPD patients treated with ICS. Thus, Sibila et al.50 raised the concern of a possible association with the use of ICS and antimicrobial drug resistant pathogens. In summary, these studies suggest that ICS may alter habitual flora and antimicrobial susceptibility particularly in COPD patients with chronic airway infections.
There are indications of ICS-interclass differences in pneumonia risk with some evidence of a weaker association of pneumonia with budesonide than with fluticasone propionate therapy. In randomized, controlled trials, treatment with fluticasone propionate alone or in combination with salmeterol was associated with increased prevalence of pneumonia compared with long-acting bronchodilator monotherapy (salmeterol or tiotropium) or placebo56,57,58,59,60. This risk appeared to increase with decreased lung function and duration of therapy73. A systematic review of 6 randomized, placebo-controlled trials tested the new formulation of fluticasone furoate alone or in combination with a new long-active β-agonist, vilanterol for at least 28 weeks of duration showed has a significant increased risk of pneumonia in ICS compared with vilanterol72,73,74. In an epidemiological study in COPD population from Canada, Suissa et al.62 reported a 101% higher risk of pneumonia in COPD patients treated with fluticasone propionate and a 17% increased risk in budesonide-treated patients when compared with controls not treated with ICSs. Most randomized controlled studies of budesonide alone or in combination with long-acting β2 agonist (formoterol) reported no or lower increased risk of pneumonia75,76,77. In a study by Sharafkhaneh et al.78 found an association between budesonide treatment and increased risk of pneumonia. In the Cochrane review by Kew and Seniukovich79, an indirect comparison found no significant difference between fluticasone propionate and budesonide monotherapy in the risk of serious adverse events (pneumonia-related or all-cause) or mortality, but a higher risk of any pneumonia event (including less serious cases treated in the community) mainly for fluticasone compared to budesonide. In the report by Halpin et al.80, an indirect comparison between budesonide and fluticasone propionate found that adverse pneumonia events and serious pneumonia adverse events were lower for budesonide. However, a retrospective analysis of the large, 4-year, prospective, randomized Understanding Potential Long-term Impacts on Function with Tiotropium (UPLIFT) trial evaluated differences in incidence of adverse respiratory events among patients entering the study on no ICS, on fluticasone propionate, or on any other ICSs, respectively81.
The data discussed suggested that there are differences in the risk of ICS formulations and pneumonia, the question is why? First, we can evaluate the pharmacokinetics and drug absorption of different ICS formulations. The use of ICS ensures that high concentration of active drug is delivered locally to the airways and lungs with a relatively low systemic burden. After inhalation, ICS are deposited as small particles on the surface of airway mucosa, and they gradually dissolve in mucosal lining fluid before they are absorbed into airway/lung tissue, target cells to exert local immunosuppression and reduction of inflammation82. The local pharmacokinetic profile of ICSs, i.e., the rate and extent of airway/pulmonary absorption, is strongly dependent on the intrinsic physicochemical properties of corticosteroids, particularly lipophilicity, aqueous solubility, and airway epithelial permeability. The important determinant of dissolution rate of ICS particles in the airway epithelial lining fluid is aqueous solubility, which greatly differs between various ICSs82. Fluticasone propionate; its long duration of action n the airways is determined by prolonged presence of slowly dissolving particles of fluticasone propionate in airway luminal fluid and the long presence of the medication within airway/lung tissue due to high lipophilicity83. On the other hand, budesonide is rapidly absorbed from the airway lumen, and in patients with COPD, a larger fraction of fluticasone was expectorated in the sputum compared with budesonide (Figure 2)84. Thus, the different ICSs molecules, their pharmacokinetics determine the duration that the compound is in the airway epithelium, these factors may impact the lung microbiota and the risk of pneumonia.
In stable COPD patients, higher airway bacterial load was shown to be significantly correlated to higher ICS dosage, and this relationship remained significant in a multivariate analysis including age, smoking status, and forced expiratory volume in 1 second (FEV1)% predicted84. Furthermore, it was shown that ICS use may alter the airway microbiota composition85,86,87,88. Importantly, according to the “keystone pathogen” hypothesis, even small alterations in the abundance of a few bacterial species can have great effects on microbial community and subsequently modify disease status. The prolonged presence of slowly dissolving particles of fluticasone propionate in the airway epithelial lining fluid compared with budesonide may cause a protracted local immunosuppression. Contoli et al.87 demonstrated that long-term use of fluticasone affects bacterial load in stable COPD patients (Figure 3). Thus, local immunosuppression by ICS may enhance susceptibility to respiratory infections and change the microbiome in the airways and lungs to allow more potential pathogenic bacteria. These changes may lead to increased risk to develop pneumonia. However, the associated impact of ICS among patients who developed pneumonia on mortality and poor clinical outcomes is a matter of significant controversy89. Some studies have demonstrated that COPD patients receiving ICS that developed pneumonia had lower mortality65,66. Further studies are needed to better understand this potentially dual effect on pneumonia due to the ICS use in patients with COPD.
Annual influenza vaccination is recommended for all adults, mainly in patients with underlying conditions such as COPD. Influenza vaccine has been shown to decrease pneumonia diagnoses, as well as related hospitalizations and cardiac events90,91,92. Current options specifically for patients 65 years of age and older include the Fluzone high-dose vaccine, which was shown to be 24% more effective in preventing flu with a standard-dose vaccine93,94,95. In COPD patients, influenza vaccination can also reduce serious illness (such as lower respiratory tract infections requiring hospitalization96 and death97,98,99. Fiore et al.97 demostrated that influenza vaccination resulted in a significnat decreased in hospitalizations due to respiratory conditions. Only few studies have evaluated the impact of influenza vaccination on COPD exacerbations and showed significant reduction in the total number of exacerbations per vaccinated subject compared with those who received placebo98. A population-based study suggested that COPD patients, particularly the elderly, had decreased risk of ischemic heart disease when they were vaccinated with influenza vaccine over subsequent years99. Thus, yearly influenza vaccination clearly provides a significant protection to COPD patients to decreased risk of hospitalization due to respiratory conditions Pneumococcal vaccines have demonstrated efficacy in preventing vaccine-strain pneumococcal pneumonia, bacteremia, and invasive disease, but do not prevent all types of CAP100. The addition of pneumococcal conjugated vaccine (PCV13) to the pediatric immunization schedule in 2010 has resulted in an indirect reduction of pneumococcal infections in adults101. The Community-Acquired Pneumonia Immunization Trial in Adults (CAPITA), a large, double-blind, randomized study, confirmed the efficacy of PCV13 in preventing vaccine-type pneumonia and invasive pneumococcal disease in adults ≥65 years of age102. In this study, PCV13 demonstrated significant efficacy in the per-protocol population protecting against first episodes of confirmed vaccine-type pneumonia and confirmed nonbacteremic and noninvasive vaccine-type pneumonia, in addition, immunogenicity studies of older adults in the United States and Europe demonstrated that conjugated vaccine generated an immune response comparable to that of polysaccharide vaccine102. Pneumococcal polysaccharide vaccine (PPV) is recommended for COPD patients 65 years and older, and in younger patients with significant comorbid conditions such as cardiac disease103. Specific data on the effects of PPV in COPD patients are limited. PPV has been shown to reduce the incidence of community-acquired pneumonia in COPD patients younger than age 65 with an FEV1 <40% predicted or comorbidities (especially cardiac comorbidities)104. A systematic review of injectable vaccines in COPD patients identified twelve randomized studies for inclusion and observed injectable polyvalent pneumococcal vaccination provides significant protection against pneumonia. The authors concluded that injectable polyvalent pneumococcal vaccination provides significant protection against pneumonia, although no evidence indicates that vaccination reduced the risk of confirmed pneumococcal pneumonia, which was a relatively rare event. Vaccination reduced the likelihood of a COPD exacerbation, and moderate-quality evidence suggests the benefits of pneumococcal vaccination in patients with COPD. Evidence was insufficient for comparison of different pneumococcal vaccine types104. Therefore, it is recommended that patients with COPD receive influenza and both pneumococcal vaccinations to prevent poor related outcomes.
COPD is the most frequent comorbid condition that is present in patients with pneumonia. These patients are older and have other co-morbidities like CVD that will further impact patients' outcomes. Human microbiome that is different in COPD patients compared with normal individuals may be impacted by medical interventions such as use of ICS. COPD and their pharmacotherapy should be considered as a risk factor for pneumonia. Furthermore, strategies to improve implementation of influenza and/or pneumococcal vaccination is critical in COPD patients at risk to develop pneumonia.
Notes
References
1. Heron M. Deaths: leading causes for 2010. Natl Vital Stat Rep 2013;62:1-96.
2. National Heart, Lung, and Blood Institute. Data fact sheet. Chronic obstructive pulmonary disease. Bethesda, MD: U.S. Department of Health and Human Services; 2003.
3. World Health Organization. The top 10 causes of death [Internet]. Geneva: World Health Organization; 2017. cited 2017 Nov 10. Available from: http://www.who.int/mediacentre/factsheets/fs310/en.
4. Jain S, Self WH, Wunderink RG, Fakhran S, Balk R, Bramley AM, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med 2015;373:415-427. PMID: 26172429.



5. Welte T, Torres A, Nathwani D. Clinical and economic burden of community-acquired pneumonia among adults in Europe. Thorax 2012;67:71-79. PMID: 20729232.


6. CDC/National Center for Health Statistics. Number and rate of discharges from short-stay hospitals and of days of care, with average length of stay and standard error, by selected first-listed diagnostic categories: United States, 2010 [Internet]. Atlanta: CDC/NCHS National Hospital Discharge Survey; 2010. cited 2017 Nov 10. Available from: http://www.cdc.gov/nchs/data/nhds/2average/2010ave2_firstlist.pdf.
7. Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2010. National Vital Statistics Reports [Internet]. Hyattsville: National Center for Health Statistics; 2013. cited 2017 Nov 10. Available from: http://www.cdc.gov/nchs/data/nvsr/nvsr61/nvsr61_04.pdf.
8. Ruiz M, Ewig S, Marcos MA, Martinez JA, Arancibia F, Mensa J, et al. Etiology of community-acquired pneumonia: impact of age, comorbidity, and severity. Am J Respir Crit Care Med 1999;160:397-405. PMID: 10430704.


9. Farr BM, Bartlett CL, Wadsworth J, Miller DL. Risk factors for community-acquired pneumonia diagnosed upon hospital admission. British Thoracic Society Pneumonia Study Group. Respir Med 2000;94:954-963. PMID: 11059948.


10. Farr BM, Sloman AJ, Fisch MJ. Predicting death in patients hospitalized for community-acquired pneumonia. Ann Intern Med 1991;115:428-436. PMID: 1872491.


11. Fine MJ, Smith MA, Carson CA, Mutha SS, Sankey SS, Weissfeld LA, et al. Prognosis and outcomes of patients with community-acquired pneumonia: a meta-analysis. JAMA 1996;275:134-141. PMID: 8531309.


12. Torres A, Serra-Batlles J, Ferrer A, Jimenez P, Celis R, Cobo E, et al. Severe community-acquired pneumonia. Epidemiology and prognostic factors. Am Rev Respir Dis 1991;144:312-318. PMID: 1859053.


13. Almirall J, Bolibar I, Balanzo X, Gonzalez CA. Risk factors for community-acquired pneumonia in adults: a population-based case-control study. Eur Respir J 1999;13:349-355. PMID: 10065680.


14. Garcia-Ordonez MA, Garcia-Jimenez JM, Paez F, Alvarez F, Poyato B, Franquelo M, et al. Clinical aspects and prognostic factors in elderly patients hospitalised for community-acquired pneumonia. Eur J Clin Microbiol Infect Dis 2001;20:14-19. PMID: 11245317.

15. Lim WS, Lewis S, Macfarlane JT. Severity prediction rules in community acquired pneumonia: a validation study. Thorax 2000;55:219-223. PMID: 10679541.



16. Lim WS, van der Eerden MM, Laing R, Boersma WG, Karalus N, Town GI, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax 2003;58:377-382. PMID: 12728155.



17. Ramirez JA, Wiemken TL, Peyrani P, Arnold FW, Kelley R, Mattingly WA, et al. Adults hospitalized with pneumonia in the United States: incidence, epidemiology, and mortality. Clin Infect Dis 2017;65:1806-1812. PMID: 29020164.



18. Chen Y, Stewart P, Dales R, Johansen H, Bryan S, Taylor G. In a retrospective study of chronic obstructive pulmonary disease inpatients, respiratory comorbidities were significantly associated with prognosis. J Clin Epidemiol 2005;58:1199-1205. PMID: 16223664.


19. Restrepo MI, Mortensen EM, Pugh JA, Anzueto A. COPD is associated with increased mortality in patients with community-acquired pneumonia. Eur Respir J 2006;28:346-351. PMID: 16611653.


20. Rello J, Rodriguez A, Torres A, Roig J, Sole-Violan J, Garnacho-Montero J, et al. Implications of COPD in patients admitted to the intensive care unit by community-acquired pneumonia. Eur Respir J 2006;27:1210-1216. PMID: 16510452.


21. Soriano JB, Visick GT, Muellerova H, Payvandi N, Hansell AL. Patterns of comorbidities in newly diagnosed COPD and asthma in primary care. Chest 2005;128:2099-2107. PMID: 16236861.


22. Mullerova H, Chigbo C, Hagan GW, Woodhead MA, Miravitlles M, Davis KJ, et al. The natural history of community-acquired pneumonia in COPD patients: a population database analysis. Respir Med 2012;106:1124-1133. PMID: 22621820.


23. Lin SH, Perng DW, Chen CP, Chai WH, Yeh CS, Kor CT, et al. Increased risk of community-acquired pneumonia in COPD patients with comorbid cardiovascular disease. Int J Chron Obstruct Pulmon Dis 2016;11:3051-3058. PMID: 27980402.



24. Feldman C, Viljoen E, Morar R, Richards G, Sawyer L, Goolam Mahomed A. Prognostic factors in severe community-acquired pneumonia in patients without co-morbid illness. Respirology 2001;6:323-330. PMID: 11844124.


25. Community-acquired pneumonia in adults in British hospitals in 1982-1983: a survey of aetiology, mortality, prognostic factors and outcome. The British Thoracic Society and the Public Health Laboratory Service. Q J Med 1987;62:195-220. PMID: 3116595.


26. Fine MJ, Orloff JJ, Arisumi D, Fang GD, Arena VC, Hanusa BH, et al. Prognosis of patients hospitalized with community-acquired pneumonia. Am J Med 1990;88:1N-8N. PMID: 2294759.
27. Fine MJ, Auble TE, Yealy DM, Hanusa BH, Weissfeld LA, Singer DE, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med 1997;336:243-250. PMID: 8995086.


28. Metlay JP, Fine MJ. Testing strategies in the initial management of patients with community-acquired pneumonia. Ann Intern Med 2003;138:109-118. PMID: 12529093.


29. Jiang HL, Chen HX, Liu W, Fan T, Liu GJ, Mao B. Is COPD associated with increased mortality and morbidity in hospitalized pneumonia? A systematic review and meta-analysis. Respirology 2015;20:1046-1054. PMID: 26177049.


32. Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998;157(5 Pt 1):1418-1422. PMID: 9603117.


33. Rose MC, Voynow JA. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol Rev 2006;86:245-278. PMID: 16371599.


34. Kesimer M, Ford AA, Ceppe A, Radicioni G, Cao R, Davis CW, et al. Airway mucin concentration as a marker of chronic bronchitis. N Engl J Med 2017;377:911-922. PMID: 28877023.



35. Kirkham S, Kolsum U, Rousseau K, Singh D, Vestbo J, Thornton DJ. MUC5B is the major mucin in the gel phase of sputum in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2008;178:1033-1039. PMID: 18776153.



36. Sibila O, Suarez-Cuartin G, Rodrigo-Troyano A, Fardon TC, Finch S, Mateus EF, et al. Secreted mucins and airway bacterial colonization in non-CF bronchiectasis. Respirology 2015;20:1082-1088. PMID: 26172851.


37. Sibila O, Garcia-Bellmunt L, Giner J, Rodrigo-Troyano A, Suarez-Cuartin G, Torrego A, et al. Airway mucin 2 is decreased in patients with severe chronic obstructive pulmonary disease with bacterial colonization. Ann Am Thorac Soc 2016;13:636-642. PMID: 26882402.



38. Braeken DC, Rohde GG, Franssen FM, Driessen JH, van Staa TP, Souverein PC, et al. Risk of community-acquired pneumonia in chronic obstructive pulmonary disease stratified by smoking status: a population-based cohort study in the United Kingdom. Int J Chron Obstruct Pulmon Dis 2017;12:2425-2432. PMID: 28860737.



39. Shukla SD, Muller HK, Latham R, Sohal SS, Walters EH. Platelet-activating factor receptor (PAFr) is upregulated in small airways and alveoli of smokers and COPD patients. Respirology 2016;21:504-510. PMID: 26662379.


40. Shukla SD, Sohal SS, Mahmood MQ, Reid D, Muller HK, Walters EH. Airway epithelial platelet-activating factor receptor expression is markedly upregulated in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2014;9:853-861. PMID: 25143722.



41. Kc R, Shukla SD, Walters EH, O'Toole RF. Temporal upregulation of host surface receptors provides a window of opportunity for bacterial adhesion and disease. Microbiology 2017;163:421-430. PMID: 28113047.


42. Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med 2008;359:2355-2365. PMID: 19038881.


43. Wilkinson TM, Patel IS, Wilks M, Donaldson GC, Wedzicha JA. Airway bacterial load and FEV1 decline in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2003;167:1090-1095. PMID: 12684248.


44. Clarridge JE 3rd. Impact of 16S rRNA gene sequence analysis for identification of bacteria on clinical microbiology and infectious diseases. Clin Microbiol Rev 2004;17:840-862. PMID: 15489351.



45. Sze MA, Dimitriu PA, Hayashi S, Elliott WM, McDonough JE, Gosselink JV, et al. The lung tissue microbiome in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012;185:1073-1080. PMID: 22427533.



46. Erb-Downward JR, Thompson DL, Han MK, Freeman CM, McCloskey L, Schmidt LA, et al. Analysis of the lung microbiome in the “healthy” smoker and in COPD. PLoS One 2011;6:e16384. PMID: 21364979.



47. Liapikou A, Polverino E, Ewig S, Cilloniz C, Marcos MA, Mensa J, et al. Severity and outcomes of hospitalised community-acquired pneumonia in COPD patients. Eur Respir J 2012;39:855-861. PMID: 21920895.


48. El-Solh AA, Sikka P, Ramadan F, Davies J. Etiology of severe pneumonia in the very elderly. Am J Respir Crit Care Med 2001;163(3 Pt 1):645-651. PMID: 11254518.


49. Arancibia F, Bauer TT, Ewig S, Mensa J, Gonzalez J, Niederman MS, et al. Community-acquired pneumonia due to gram-negative bacteria and pseudomonas aeruginosa: incidence, risk, and prognosis. Arch Intern Med 2002;162:1849-1858. PMID: 12196083.


50. Sibila O, Rodrigo-Troyano A, Shindo Y, Aliberti S, Restrepo MI. Multidrug-resistant pathogens in patients with pneumonia coming from the community. Curr Opin Pulm Med 2016;22:219-226. PMID: 26960007.


51. Niederman MS, Mandell LA, Anzueto A, Bass JB, Broughton WA, Campbell GD, et al. Guidelines for the management of adults with community-acquired pneumonia: diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am J Respir Crit Care Med 2001;163:1730-1754. PMID: 11401897.


52. Global Initiative for Asthma. Global strategy for asthma management and prevention [Internet]. Global Initiative for Asthma; 2017. cited 2017 Nov 10. Available from: http://www.ginasthma.org/.
53. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of COPD [Internet]. Global Initiative for Chronic Obstructive Lung Disease; 2017. cited 2017 Nov 10. Available from: http://www.goldcopd.org/.
54. Calverley P, Pauwels R, Vestbo J, Jones P, Pride N, Gulsvik A, et al. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet 2003;361:449-456. PMID: 12583942.


55. Nannini L, Cates CJ, Lasserson TJ, Poole P. Combined corticosteroid and long-acting beta-agonist in one inhaler versus placebo for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2007;(4):CD003794. PMID: 17943798.



56. Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 2007;356:775-789. PMID: 17314337.


57. Kardos P, Wencker M, Glaab T, Vogelmeier C. Impact of salmeterol/fluticasone propionate versus salmeterol on exacerbations in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2007;175:144-149. PMID: 17053207.


58. Wedzicha JA, Calverley PM, Seemungal TA, Hagan G, Ansari Z, Stockley RA, et al. The prevention of chronic obstructive pulmonary disease exacerbations by salmeterol/fluticasone propionate or tiotropium bromide. Am J Respir Crit Care Med 2008;177:19-26. PMID: 17916806.


59. Ernst P, Gonzalez AV, Brassard P, Suissa S. Inhaled corticosteroid use in chronic obstructive pulmonary disease and the risk of hospitalization for pneumonia. Am J Respir Crit Care Med 2007;176:162-166. PMID: 17400730.


60. Welte T, Miravitlles M, Hernandez P, Eriksson G, Peterson S, Polanowski T, et al. Efficacy and tolerability of budesonide/formoterol added to tiotropium in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2009;180:741-750. PMID: 19644045.


61. Dransfield MT, Bourbeau J, Jones PW, Hanania NA, Mahler DA, Vestbo J, et al. Once-daily inhaled fluticasone furoate and vilanterol versus vilanterol only for prevention of exacerbations of COPD: two replicate double-blind, parallel-group, randomised controlled trials. Lancet Respir Med 2013;1:210-223. PMID: 24429127.


62. Suissa S, Patenaude V, Lapi F, Ernst P. Inhaled corticosteroids in COPD and the risk of serious pneumonia. Thorax 2013;68:1029-1036. PMID: 24130228.



63. DiSantostefano RL, Sampson T, Le HV, Hinds D, Davis KJ, Bakerly ND. Risk of pneumonia with inhaled corticosteroid versus long-acting bronchodilator regimens in chronic obstructive pulmonary disease: a new-user cohort study. PLoS One 2014;9:e97149. PMID: 24878543.



64. Festic E, Scanlon PD. Incident pneumonia and mortality in patients with chronic obstructive pulmonary disease: a double effect of inhaled corticosteroids? Am J Respir Crit Care Med 2015;191:141-148. PMID: 25409118.



65. Chen D, Restrepo MI, Fine MJ, Pugh MJ, Anzueto A, Metersky ML, et al. Observational study of inhaled corticosteroids on outcomes for COPD patients with pneumonia. Am J Respir Crit Care Med 2011;184:312-316. PMID: 21512168.



66. Malo de Molina R, Mortensen EM, Restrepo MI, Copeland LA, Pugh MJ, Anzueto A. Inhaled corticosteroid use is associated with lower mortality for subjects with COPD and hospitalised with pneumonia. Eur Respir J 2010;36:751-757. PMID: 20413535.


67. Joo MJ, Au DH, Fitzgibbon ML, Lee TA. Inhaled corticosteroids and risk of pneumonia in newly diagnosed COPD. Respir Med 2010;104:246-252. PMID: 19879745.


68. Sellares J, Lopez-Giraldo A, Lucena C, Cilloniz C, Amaro R, Polverino E, et al. Influence of previous use of inhaled corticoids on the development of pleural effusion in community-acquired pneumonia. Am J Respir Crit Care Med 2013;187:1241-1248. PMID: 23590264.


69. Stuck AE, Minder CE, Frey FJ. Risk of infectious complications in patients taking glucocorticosteroids. Rev Infect Dis 1989;11:954-963. PMID: 2690289.



70. Conesa D, Rello J, Valles J, Mariscal D, Ferreres JC. Invasive aspergillosis: a life-threatening complication of short-term steroid treatment. Ann Pharmacother 1995;29:1235-1237. PMID: 8672828.


71. Wiest PM, Flanigan T, Salata RA, Shlaes DM, Katzman M, Lederman MM. Serious infectious complications of corticosteroid therapy for COPD. Chest 1989;95:1180-1184. PMID: 2721249.


72. Sibila O, Laserna E, Mortensen EM, Anzueto A, Restrepo MI. Effects of inhaled corticosteroids on pneumonia severity and antimicrobial resistance. Respir Care 2013;58:1489-1494. PMID: 23345471.



73. Jenkins CR, Jones PW, Calverley PM, Celli B, Anderson JA, Ferguson GT, et al. Efficacy of salmeterol/fluticasone propionate by GOLD stage of chronic obstructive pulmonary disease: analysis from the randomised, placebo-controlled TORCH study. Respir Res 2009;10:59. PMID: 19566934.



74. Rodrigo GJ, Neffen H. A systematic review with meta-analysis of fluticasone furoate/vilanterol combination for the treatment of stable COPD. Pulm Pharmacol Ther 2017;42:1-6. PMID: 27864038.


75. Calverley PM, Boonsawat W, Cseke Z, Zhong N, Peterson S, Olsson H. Maintenance therapy with budesonide and formoterol in chronic obstructive pulmonary disease. Eur Respir J 2003;22:912-919. PMID: 14680078.


76. Rennard SI, Tashkin DP, McElhattan J, Goldman M, Ramachandran S, Martin UJ, et al. Efficacy and tolerability of budesonide/formoterol in one hydrofluoroalkane pressurized metered-dose inhaler in patients with chronic obstructive pulmonary disease: results from a 1-year randomized controlled clinical trial. Drugs 2009;69:549-565. PMID: 19368417.



77. Janson C, Larsson K, Lisspers KH, Stallberg B, Stratelis G, Goike H, et al. Pneumonia and pneumonia related mortality in patients with COPD treated with fixed combinations of inhaled corticosteroid and long acting beta2 agonist: observational matched cohort study (PATHOS). BMJ 2013;346:f3306. PMID: 23719639.



78. Sharafkhaneh A, Southard JG, Goldman M, Uryniak T, Martin UJ. Effect of budesonide/formoterol pMDI on COPD exacerbations: a double-blind, randomized study. Respir Med 2012;106:257-268. PMID: 22033040.


79. Kew KM, Seniukovich A. Inhaled steroids and risk of pneumonia for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2014;(3):CD010115. PMID: 24615270.



80. Halpin DM, Gray J, Edwards SJ, Morais J, Singh D. Budesonide/formoterol vs. salmeterol/fluticasone in COPD: a systematic review and adjusted indirect comparison of pneumonia in randomised controlled trials. Int J Clin Pract 2011;65:764-774. PMID: 21676119.


81. Morjaria JB, Rigby A, Morice AH. Inhaled corticosteroid use and the risk of pneumonia and COPD exacerbations in the UPLIFT Study. Lung 2017;195:281-288. PMID: 28255905.




82. Edsbacker S, Wollmer P, Selroos O, Borgstrom L, Olsson B, Ingelf J. Do airway clearance mechanisms influence the local and systemic effects of inhaled corticosteroids? Pulm Pharmacol Ther 2008;21:247-258. PMID: 17950641.


83. Johnson M. Pharmacodynamics and pharmacokinetics of inhaled glucocorticoids. J Allergy Clin Immunol 1996;97(1 Pt 2):169-176. PMID: 8568148.


84. Dalby C, Polanowski T, Larsson T, Borgstrom L, Edsbacker S, Harrison TW. The bioavailability and airway clearance of the steroid component of budesonide/formoterol and salmeterol/fluticasone after inhaled administration in patients with COPD and healthy subjects: a randomized controlled trial. Respir Res 2009;10:104. PMID: 19878590.



85. Garcha DS, Thurston SJ, Patel AR, Mackay AJ, Goldring JJ, Donaldson GC, et al. Changes in prevalence and load of airway bacteria using quantitative PCR in stable and exacerbated COPD. Thorax 2012;67:1075-1080. PMID: 22863758.


86. Pragman AA, Kim HB, Reilly CS, Wendt C, Isaacson RE. The lung microbiome in moderate and severe chronic obstructive pulmonary disease. PLoS One 2012;7:e47305. PMID: 23071781.



87. Contoli M, Pauletti A, Rossi MR, Spanevello A, Casolari P, Marcellini A, et al. Long-term effects of inhaled corticosteroids on sputum bacterial and viral loads in COPD. Eur Respir J 2017;50:1700451PMID: 28982774.


88. Talbot HK, Zhu Y, Chen Q, Williams JV, Thompson MG, Griffin MR. Effectiveness of influenza vaccine for preventing laboratory-confirmed influenza hospitalizations in adults, 2011-2012 influenza season. Clin Infect Dis 2013;56:1774-1777. PMID: 23449269.




89. Sibila O, Suarez-Cuartin G, Rodrigo-Troyano A, Anzueto A. Corticosteroids and pneumonia in chronic obstructive pulmonary disease: a dual effect? BRN Rev 2015;1:105-115.
90. Udell JA, Zawi R, Bhatt DL, Keshtkar-Jahromi M, Gaughran F, Phrommintikul A, et al. Association between influenza vac cination and cardiovascular outcomes in high-risk patients: a meta-analysis. JAMA 2013;310:1711-1720. PMID: 24150467.


91. Centers for Disease Control and Prevention. Fluzone highdose seasonal influenza vaccine [Internet]. Atlanta: Centers for Disease Control and Prevention; 2017. cited 2016 Nov 30. Available from: http://www.cdc.gov/flu/protect/vaccine/qa_fluzone.htm.
92. DiazGranados CA, Dunning AJ, Kimmel M, Kirby D, Treanor J, Collins A, et al. Efficacy of high-dose versus standarddose influenza vaccine in older adults. N Engl J Med 2014;371:635-645. PMID: 25119609.


93. Centers for Disease Control and Prevention. FLUAD flu vaccine with adjuvant [Internet]. Atlanta: Centers for Disease Control and Prevention; 2017. cited 2016 Nov 30. Available from: http://www.cdc.gov/flu/protect/vaccine/adjuvant.htm.
94. Wongsurakiat P, Maranetra KN, Wasi C, Kositanont U, Dejsomritrutai W, Charoenratanakul S. Acute respiratory illness in patients with COPD and the effectiveness of influenza vaccination: a randomized controlled study. Chest 2004;125:2011-2020. PMID: 15189916.


95. Poole PJ, Chacko E, Wood-Baker RW, Cates CJ. Influenza vaccine for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2006;(1):CD002733. PMID: 16437444.


96. Nichol KL, Margolis KL, Wuorenma J, Von Sternberg T. The efficacy and cost effectiveness of vaccination against influenza among elderly persons living in the community. N Engl J Med 1994;331:778-784. PMID: 8065407.


97. Fiore AE, Shay DK, Broder K, Iskander JK, Uyeki TM, Mootrey G, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep 2009;58:1-52.
98. Huang CL, Nguyen PA, Kuo PL, Iqbal U, Hsu YH, Jian WS. Influenza vaccination and reduction in risk of ischemic heart disease among chronic obstructive pulmonary elderly. Comput Methods Programs Biomed 2013;111:507-511. PMID: 23769164.


99. Kobayashi M, Bennett NM, Gierke R, Almendares O, Moore MR, Whitney CG, et al. Intervals Between PCV13 and PPSV23 vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2015;64:944-947. PMID: 26334788.


100. Bonten MJ, Huijts SM, Bolkenbaas M, Webber C, Patterson S, Gault S, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med 2015;372:1114-1125. PMID: 25785969.


101. Jackson LA, Gurtman A, Rice K, Pauksens K, Greenberg RN, Jones TR, et al. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine in adults 70 years of age and older previously vaccinated with 23-valent pneumococcal polysaccharide vaccine. Vaccine 2013;31:3585-3593. PMID: 23688527.


102. Tomczyk S, Bennett NM, Stoecker C, Gierke R, Moore MR, Whitney CG, et al. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2014;63:822-825. PMID: 25233284.


103. Alfageme I, Vazquez R, Reyes N, Munoz J, Fernandez A, Hernandez M, et al. Clinical efficacy of anti-pneumococcal vaccination in patients with COPD. Thorax 2006;61:189-195. PMID: 16227328.


104. Walters JA, Tang JN, Poole P, Wood-Baker R. Pneumococcal vaccines for preventing pneumonia in chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2017;1:CD001390. PMID: 28116747.


Figure 1
The impact of comorbid conditions on the incidence of patients hospitalized with community-acquired pneumonia. CHF: congestive heart failure; COPD: chronic obstructive pulmonary disease. Reproduced from Ramirez et al. Clin Infect Dis 2017;65:1806-12, with permission of Oxford University Press17.
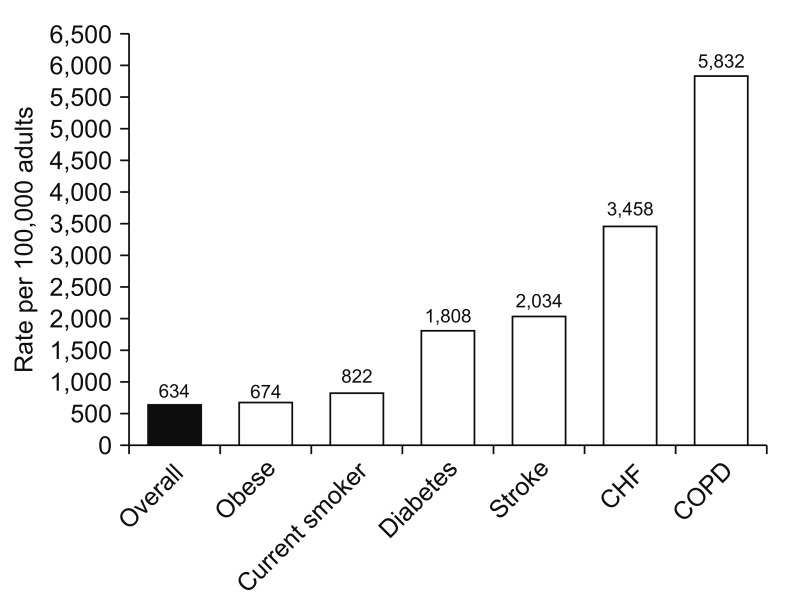
Figure 2
Cumulative mean amounts of expectorated sputum (A) and budesonide and fluticasone propionate (B) over 6-hour collection after inhalation of a dose of salmeterol/fluticasone propionate (50/500 µg via Diskus; GlaxoSmithKline, Brentford, UK) or budesonide/formoterol (400/12 µg via Turbuhaler; AstraZeneca, Gothenburg, Sweden). Mean value plots of the amount of expectorated sputum (arithmetic means) (A) and budesonide and fluticasone propionate in the expectorated sputum (percentage of estimated lung-deposited dose, geometric means) (B), cumulative over the 6-hour collection period. BUD/FORM: budesonide/formoterol; SAL/FLU: salmeterol/fluticasone propionate. Reproduced from Dalby et al. Respir Res 2009;10:104, according to the Creative Commons license BMC84.
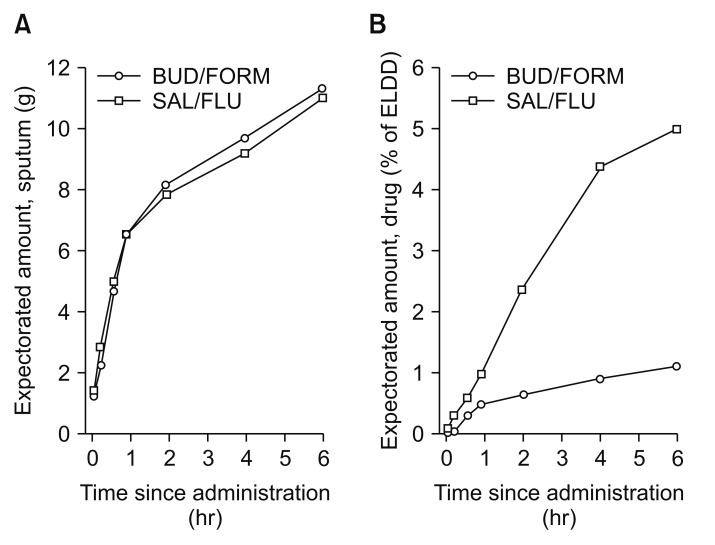
Figure 3
Airway bacterial load and microbiome analysis. Total bacterial load is shown as colony-forming units (CFU) per mL and was assessed at baseline (V0) and after 12 months of therapy (V4) in sputum samples from patients in both the salmeterol/fluticasone (SALM/FP) and SALM alone groups. Reproduced from Contoli et al. Eur Respir J 2017;50:1700451, with permission of European Respiratory Society87.
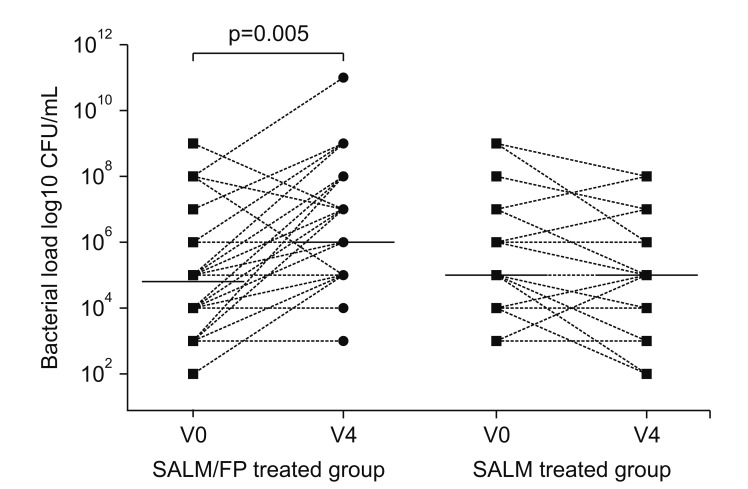
Table 1
Factors that may predispose Pneumonia in COPD patients
| Chronic bronchitis |
| Persistent mucus production |
| Presence of bacterial colonization |
| Microbioma imbalances |
| Increased airway inflammation |
| Impaired host immunity |
| Structural damage |
Table 2
Studies evaluating the effects of inhaled corticosteroids in COPD patients and the risk of pneumonia
| Author/Year | Study design | No. of COPD patients | Type of corticosteroid | Risk of pneumonia |
|---|---|---|---|---|
| Kardos et al.57 (2007) | Randomized controlled trial | 994 | Fluticasone propionate | Increased risk |
| Calverley et al.56 (2007) | Randomized controlled trial | 6,112 | Fluticasone propionate | Increased risk |
| Wedzicha et al.58 (2008) | Randomized controlled trial | 1,323 | Fluticasone propionate | Increased risk |
| Ernst et al.59 (2007) | Case-control study | 175,906 | Beclomethasone, budesonide, triamcinolone,fluticasone and flunisolide | Increased risk |
| Welte et al.60 (2009) | Randomized controlled trial | 660 | Budesonide No | increased risk |
| Mullerova et al.22 (2012) | Cohort study | 40,414 | Not specified | Increased risk |
| Dransfield et al.61 (2013) | Two parallel-group randomized controllled trials | 3,255 | Fluticasone furoate | Increased risk |
| Suissa et al.62 (2013) | Cohort study | 163,514 | Beclomethasone, budesonide, fluticasone, triamcinolone and flunisolide | Increased risk |
| DiSantostefano et al.63 (2014) | Cohort study | 11,555 | Not specified | Increased risk |



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Print
Print Download Citation
Download Citation




