Stridor in a Patient with Uncontrolled Diabetes: An Uncommon Adversary, Successfully Managed with Bronchoscopy
Article information
Invasive mucormycosis is a fatal opportunistic infection caused by fungi from the Mucoraceae family. Uncontrolled diabetes mellitus and post-organ transplant status are two of the strongest risk factors for invasive mucormycosis. Rhino-cerebral, renal and pulmonary involvement are most commonly seen [1]. Isolated invasion of the trachea and larynx can lead to life-threatening airway obstruction; however, they are rare manifestations of invasive mucormycosis [2]. Diagnosis is based on histopathology as culture positivity is uncommon and has poor sensitivity. Liposomal amphotericin B is the recommended first-choice drug for the treatment of invasive mucormycosis while posaconazole is used as a salvage therapy. Due to vaso-invasive nature of the disease, surgery is recommended following drug treatment [1]. In this manuscript, we report our experience in conservatively managing a patient with isolated tracheal mucormycosis.
A 41-year-old lady with uncontrolled type 2 diabetes mellitus presented at our emergency room with stridor, dysphagia and progressive breathlessness which had persisted for 1 month. On examination, she had biphasic stridor. Her hematological investigation results showed unremarkable except for the leukocytosis (13,400/μL) and hyperglycemia (556 mg/dL) with positive urinary ketones. Her blood gas analysis results were suggestive of high anion gap metabolic acidosis. Her hemoglobin A1c was 16.7%. Chest radiography was largely unremarkable. A syndromic diagnosis of diabetic ketoacidosis was made. She was managed with insulin infusion and oxygen supplementation with intravenous fluids and antibiotics (piperacillin-tazobactam). For evaluation of the stridor, a contrast-enhanced computed tomography (CECT) of the neck and thorax was done. The CECT showed peripherally enhancing circumferential collection around the trachea of size 4.3×2.4 cm and with specks of air pockets (Figure 1A), and extending from C7 to T2 vertebral levels. Notably, the rest of the lung parenchyma was normal. She underwent a flexible-bronchoscopy examination which revealed the presence of a thick purulent looking circumferential membranous layer (Figure 1C), adherent to the underlying unhealthy mucosa, extending from the second tracheal ring to 3 cm above the carina and causing 70% to 80% of luminal narrowing. Given the critical stenosis and thick adherent pseudomembrane formation, rigid bronchoscopy was planned. During rigid-bronchoscopy, after sedation, the patient was intubated using a size 12 tracheoscope by Karl Storz with a 4K Ultra-High-Definition camera system (Tuttlingen, Germany). After reaching the narrowing, the scope was negotiated into the lower end of the trachea. Once the ventilation was established, the scope was pulled back up to the upper end of stenosis and the pseudomembrane was removed using alligator tooth optical forceps. Significant pull strength was required to peal the pseudomembranes from their bases. Circumferential rings of the thick-organized pseudomembrane were completely removed. After multiple attempts, the entire visible pseudomembrane was removed. Stenosis, which was due to edematous unhealthy mucosa, was reduced to 10% to 20%. Post-procedure, the patient was extubated on the operation table and shifted to the intensive care unit. There was a significant reduction in respiratory distress and audible stridor. A sample of the pseudomembrane was sent for microbiological culture (bacterial, fungal, and mycobacterial culture), histopathology, and tubercular workup (staining and cartridge-based nucleic acid amplification test).
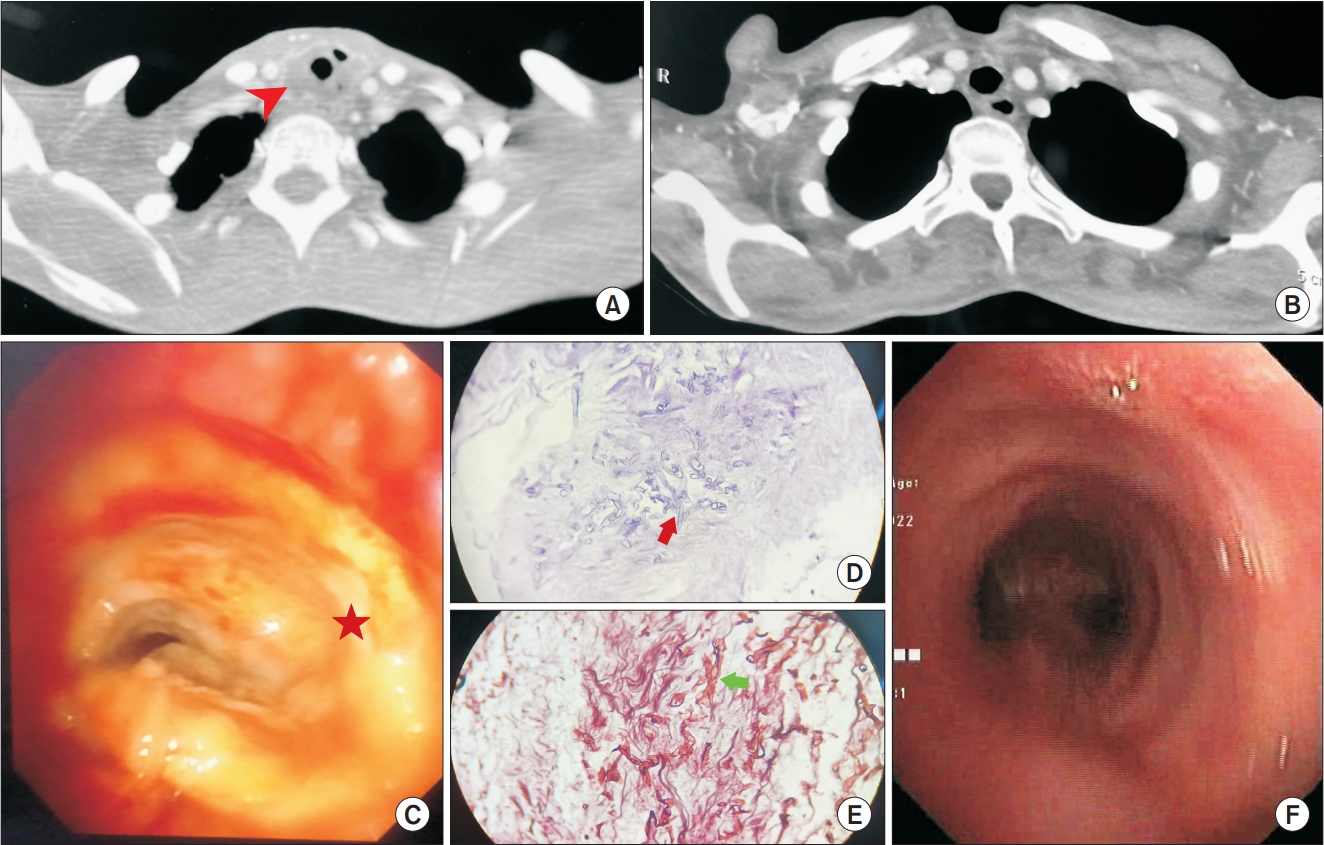
(A) Contrast-enhanced computed tomography (CECT) of the thorax showing hypoattenuating circumferential collection (arrowhead) around the trachea with a few air specks. (B) Follow-up CECT thorax showing near complete resolution of the collection. (C) Bronchoscopic image of the whitish creamy slough (star) causing critical narrowing of the trachea. (D) Hematoxylin & eosin stain showing irregular thick fungal hyphae in the necrotic background (arrow). (E) Gomori methamine stain (400×) revealing broad aseptate hyphae with acute angle branching (arrow). (F) Bronchoscopic images showing near complete recovery following removal of the slough under rigid-bronchoscopy and complete course of liposomal amphotericin B treatment.
Microbiological and tubercular workup were negative. However, histopathologic examination showed the presence of broad aseptate hyphae with acute angle branching and tissue invasion suggestive of mucormycosis (Figure 1D, E). As clinical and histopathologic pictures were consistent with mucormycosis, intravenous-liposomal-amphotericin B infusion at 5 mg/kg was started. Electrolytes and renal function tests were monitored daily. During amphotericin B treatment, there were multiple episodes of hypokalemia which were corrected enterally as well as intravenously as and when recommended according to the guidelines. Blood sugar was controlled with insulin. After 1 week of therapy, she underwent flexible bronchoscopy examination which showed the presence of a minor residual membrane on the mid tracheal wall, it was removed using forceps. Retained secretions were removed by toileting with normal saline. After 20 days of treatment with liposomal amphotericin B, CECT neck and thorax showed complete resolution of the peritracheal collection (Figure 1B). Subsequently, amphotericin B was stopped and posaconazole maintenance treatment was initiated for the next 6 weeks. After a 1-year follow-up, the patient is doing fine with no recurrence and with a near normal tracheobronchial tree, which was confirmed with interval flexible-bronchoscopy examination (Figure 1F).
Tracheal or upper airway mucormycosis is a rare entity. The usual presentation is in the form of respiratory distress and stridor requiring urgent tracheostomy or a more invasive forms of surgeries like tracheal resection. Imaging findings are present in most cases but not characteristic. For a complete cure, multimodal management is recommended, which includes amphotericin infusions followed by curative resection [2-6]. Conservative management with only amphotericin and bronchoscopic removal of slough has also been reported to be successful [7,8]. In a systematic review, diabetes mellitus was found to be the most common risk factor for mucormycosis, with the majority of the patients receiving amphotericin B and an overall case fatality rate of 50% [9]. Majority of deaths reported were due to massive hemoptysis or respiratory failure. Bronchoscopy with endobronchial biopsy histology established diagnosis in most cases. Better outcome was observed when bronchoscopy-guided or surgical interventions were combined with amphotericin B [8]. The probable mechanism behind uncontrolled diabetes as a high risk for developing invasive mucormycosis is neutrophilic dysfunction [10]. Low threshold for suspicions in at-risk subjects, histopathologic confirmation and aggressive management with antifungals along with bronchoscopic debridement may improve outcome.
In this case, we successfully managed tracheal mucormycosis in a patient with uncontrolled type 2 diabetes mellitus with bronchoscopy.
Informed consent was obtained from the patient. Ethical principles of publication were adhered to during writing of this manuscript.
Notes
Authors’ Contributions
Conceptualization: Singh PK. Methodology: Gulia K, Singh PK. Validation: Singhal S, Chaudhry D. Investigation: Jain P, Ahuja A. Writing - original draft preparation: Gulia K, Ahuja A. Writing - review and editing: Chaudhry D, Singh PK. Approval of final manuscript: all authors.
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Funding
No funding to declare.
Acknowledgements
We thank the index subjects for the consent to allow us to publish the case report.
