Determinants of Willingness to Undergo Lung Cancer Screening among High-Risk Current and Ex-smokers in Sabah, Malaysia: A Cross-Sectional Pilot Study
Article information
Abstract
Background
Attitudes towards smoking, lung cancer screening, and perceived risk of lung cancer have not been widely studied in Malaysia. The primary objective of this study was to describe the factors affecting the willingness of high-risk current smokers and ex-smokers to undergo low-dose computed tomography (LDCT) screening for lung cancer.
Methods
A prospective, cross-sectional questionnaire study was conducted in current smokers or ex-smokers aged between 55 and 80 years at three hospitals in Kota Kinabalu, Sabah, Malaysia. The questionnaire recorded the following parameters: perceived lung cancer risk; Prostate Lung Colon Ovarian Cancer 2012 risk prediction model excluding race and ethnicity predictor (PLCOm2012norace); demographic characteristics; psychosocial characteristics; and attitudes towards lung cancer and lung cancer screening.
Results
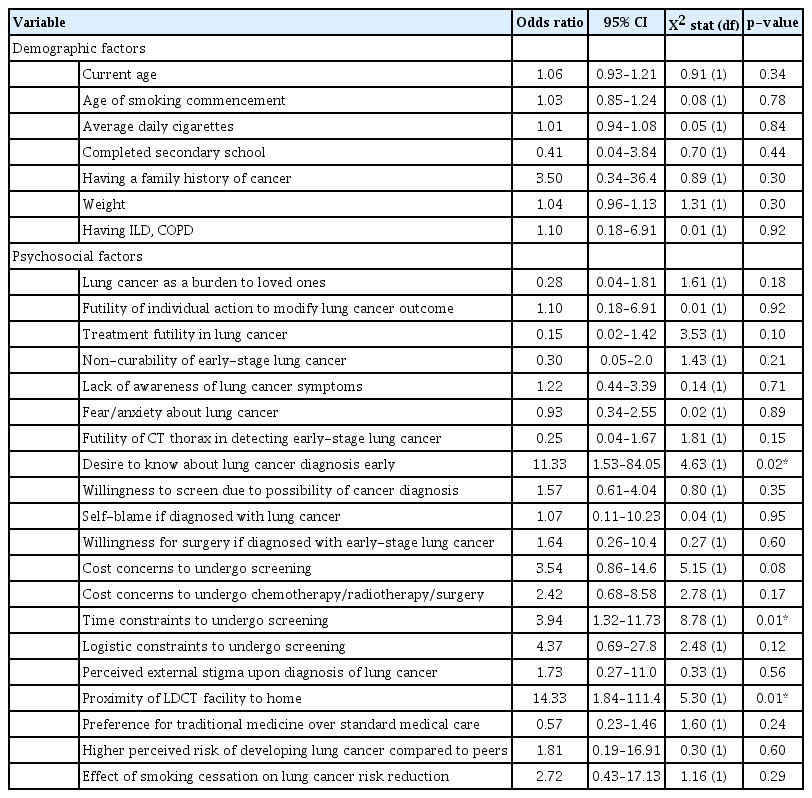
A vast majority of the 95 respondents (94.7%) indicated their willingness to undergo screening. Stigma of lung cancer, low levels of knowledge about lung cancer symptoms, concerns about financial constraints, and a preference for traditional medication were still prevalent among the respondents, and they may represent potential barriers to lung cancer screening uptake. A desire to have an early diagnosis (odds ratio [OR], 11.33; 95% confidence interval [CI], 1.53 to 84.05; p=0.02), perceived time constraints (OR, 3.94; 95% CI, 1.32 to 11.73; p=0.01), and proximity of LDCT screening facilities (OR, 14.33; 95% CI, 1.84 to 111.4; p=0.01) had significantly higher odds of willingness to undergo screening.
Conclusion
Although high-risk current smokers and ex-smokers are likely to undergo screening for lung cancer, several psychosocial barriers persist. The results of this study may guide the policymakers and clinicians regarding the need to improve lung cancer awareness in our population.
Introduction
Lung cancer has a 5-year relative survival rate of 11% in Malaysia due to late-stage diagnosis, with 90% of lung cancer patients in Malaysia diagnosed with either stage III or IV disease [1]. Meta-analysis of nine randomized control trials demonstrated a 16% relative reduction in lung cancer mortality on low-dose computed tomography (LDCT) screening [2]. In Malaysia, there are no national lung cancer screening programs for high-risk individuals, and LDCT screening for lung cancer is offered only in private hospitals [3].
Any screening program should first understand its participants. Previous studies have indicated low levels of knowledge of lung cancer symptoms and low perceived risk of lung cancer among high-risk Malaysians [4,5]. However, both these studies were single center studies conducted in the more urban, prosperous regions of West Malaysia. In contrast, the two states of Sabah and Sarawak, which constitute East Malaysia, remain the most impoverished states with relatively larger proportions of rural populations and suboptimal infrastructure [6]. It remains unclear if high-risk individuals in Sabah, East Malaysia would be more willing to undergo lung cancer screening if community-based screening was provided, owing to the local topographical and geographical challenges.
The primary objective of this study was to describe the effects of (1) perceived lung cancer risk; Prostate Lung Colon Ovarian Cancer 2012 risk prediction model excluding race and ethnicity predictor (PLCOm2012norace); (2) demographic characteristics; (3) psychosocial characteristics; and (4) attitudes towards lung cancer and lung cancer screening on the willingness to undergo LDCT screening for lung cancer.
Materials and Methods
1. Study design and participants
This was a cross-sectional, interviewer-administered questionnaire study of current or former smokers presenting as inpatients to our Medical and Respiratory Units of the respective study sites. Potential respondents were opportunistically identified by the investigators and invited to participate in the study if they fulfilled the inclusion criteria. The questionnaire was made available in both English and Malay languages.
The study was conducted in two public hospitals and one private hospital located in Kota Kinabalu, Sabah, Malaysia. Recruitment and data collection were carried out between May 2022 to November 2022. We included individuals aged 55 to 80 years, who were not known to have lung cancer, and were current smokers with a history of smoking minimum 20 pack-years or ex-smokers with a history of smoking minimum 20 pack-years and had stopped smoking within the last 15 years. The minimum age of 55 years was determined, as the objective risk calculator, PLCOm2012norace model, only accounted for individuals who were aged 55 years and above [7]. Meanwhile, the smoking criteria were adapted from the United States Preventive Services Task Force (USPSTF) criteria [8]. We excluded individuals with a known lung cancer diagnosis, and those who did not fulfill the age or smoking criterion described above.
2. Bilingual questionnaire design and validation
The questionnaire was adapted from two validated questionnaires, with permission from their respective authors. The two validated questionnaires consisted of a study comparing the absolute and perceived risk [9], and another study on the psychological determinants of lung screening uptake [10]. Malay translation was performed as follows: the content was qualitatively validated in terms of relevance, importance, and coverage by three authors who were subject-matter experts. Bilingual comprehensibility and accuracy were ascertained by forward-backward translation. Forward translation from English to Malay was separately and independently conducted by a linguistic expert and subject-matter expert, followed by backward translation from Malay to English by a linguistic expert and subject-matter expert. Then, three authors (Huan NC, Rosli T, and Ramarmuty HYD) reviewed the separate versions of the questionnaire and finalized the pre-final translated version in Malay. Face validation was then conducted with five participants, and two authors (Nyanti LE and Rosli T) reviewed and finalized the final translated version in Malay.
3. Questionnaire content
The questionnaire was divided into three sections, with a total of 34 questions. Section one consisted of 13 questions covering the demographics, smoking history, perceived lung cancer risk, and objective lung cancer risk, and it was adapted with permission from a validated survey from an Australian study [9]. Minor changes were made to the question on race and ethnicity to reflect Malaysian racial demographics. Questions on objective lung cancer risk were derived using the Prostate Lung Colon Ovarian Cancer 2012 risk prediction model excluding the race and ethnicity predictor (PLCOm2012norace) [8]. A 1.5% risk threshold was used, as it was the threshold at which the LDCT mortality reduction benefits over a chest radiograph began [11].
Section two presents an overview of LDCT screening, including benefits and harms to the respondent, and it was also adapted with permission from the Australian study [9]. Section three consisted of 21 questions adapted from the Self-Regulatory Questionnaire for Lung Cancer Screening (SQR-LCS) questionnaire [10], which is a validated measure of psychological factors that affect lung cancer screening uptake. The scope of the questions encompassed several psychological constructs, such as consequences (question 14), personal control (question 15), treatment control (question 16), illness coherence (question 18), emotional representation (question 19), behavioral response and appraisal (questions 20, 21, and 22), risk perception (question 32), response efficacy for smoking cessation (question 33), treatment intention (question 24), perceived stigma (question 23), and lung cancer survival (question 17). Additional questions not covered in either prior studies (questions 10 and 11) were integrated; these were questions related to the local Malaysian setting, such as costs incurred for screening and treatment of lung cancer (questions 25 and 26), perceived time burden to undergo screening (question 27), perceived logistical burden to undergo screening (questions 28 and 29), perceived community stigma towards lung cancer (question 30), and cultural beliefs related to cancer treatment (question 31). Willingness of the respondent to undergo lung cancer screening was indicated in the final question (question 34).
4. Statistical analysis
The sample size was calculated according to the prevalence formula [12], which was derived from a smoker prevalence of 13% in Sabah state, as reported in the National Health and Morbidity Survey (NHMS) 2019 [13]. We used a z value of 1.96, corresponding to a confidence coefficient of 0.95; and a precision, or margin of error of 7.5%. The minimum sample size needed to achieve a 7.5% precision in estimating the prevalence was 77 subjects.
Demographic characteristics were presented using descriptive analyses, namely frequencies, percentages, means and standard deviations, or medians and interquartile ranges. Group comparisons between patients who preferred and did not prefer screening were performed using the chi-squared test, t-test, or Wilcoxon Signed Rank test, as appropriate. A p-value <0.05 was considered significant. Missing data were not imputed. Analysis was performed using SPSS version 28 (IBM Co., Armonk, NY, USA).
5. Ethics approval
This study received ethics approval from the Medical Research & Ethics Committee, Ministry of Health Malaysia (NMRR ID-22-00669-QWZ). Written informed consent was obtained from all patients.
Results
1. Demographic and smoking characteristics
We interviewed a total of 95 respondents with a mean age of 63.3 years, and they were predominantly of male gender (n=92) and Sabahan native ethnicity (n=44). Overall, our respondents had a low to moderate level of education, with nearly two-thirds not having completed secondary school and nearly one-third who completed secondary school but did not progress further. Onefourth of the respondents had chronic obstructive pul-monary disease (COPD), while the prevalence of past tuberculosis infection was 16.8% (n=16). More than half of the respondents were overweight. There were only two respondents from the private hospital. The demographic details are presented in Table 1.

Demographic characteristics, smoking history, and perceived and absolute risk of the study participants
The earliest age of smoking commencement was 5 years, while the median age of smoking commencement and total duration of smoking was 17.5 and 41 years, respectively. One-third (n=32) of the respondents were current smokers, while the remaining twothirds were ex-smokers; with a median daily cigarette consumption of 20 cigarettes, and median exposure pack-years of 42 years. Among the ex-smokers, median years since quitting smoking was 6 years, but seven individuals (12.5%) conceded that they might start smoking again. A vast majority of current smokers expressed their intention to quit (n=27, 84.3%), but they varied in terms of the actions taken. Meanwhile, five current smokers had no intention of smoking cessation. Further data on the smoking history and perceptions are detailed in Table 1.
2. Absolute and perceived risk
Among our respondents, the median 6-year risk of developing lung cancer was 2.6% (Table 1). Interestingly, 18 respondents (18.9%) had a risk of less than 1.5%, which made them ineligible for screening. The risk-reducing characteristics of these 18 respondents, included having smoked less in terms of the duration and quantity of cigarettes, a higher body mass index, absence of COPD, absence of personal or family history of other cancers, being a former smoker, and a higher level of education.
In terms of perceived risk, the majority of the respondents perceived themselves to be at an equal or lower risk of developing lung cancer compared to non-smoking individuals of the same age and gender. A greater proportion of these respondents were eligible for screening compared to those who perceived themselves to be at higher risk (Figure 1). Only one-third (n=29) of the respondents perceived themselves to be at higher risk of developing lung cancer; out of these, one-third had a PLCOm2012norace 6-year risk of less than 1.5%, indicating that they may not actually require screening.
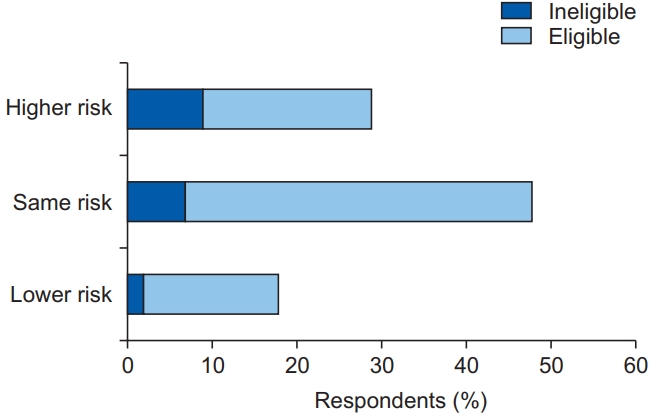
Eligibility for screening based on the absolute risk (perceived lung cancer risk and objective risk [PLCOm2012norace] >1.5%) in the higher, same, and lower perceived risk groups.
Five respondents expressed their unwillingness to undergo LDCT screening. One of these five respondents demonstrated concurrence between the absolute risk (PLCOm2012norace 6-year risk: 1.0%) and perceived risk; having accurately perceived himself to be at a lower risk than a non-smoking individual of the same age and gender. The remaining four respondents were eligible for screening, with a PLCOm2012norace 6-year risk of 9.0%, 2.2%, 4.7%, and 1.9%, respectively. However, only one respondent perceived himself to be at a higher risk. The respondent with the highest absolute risk of 9.0% perceived himself to be at an equal risk of developing lung cancer compared to a non-smoking individual of the same age and gender.
3. Attitudes towards lung cancer screening, diagnosis, and management
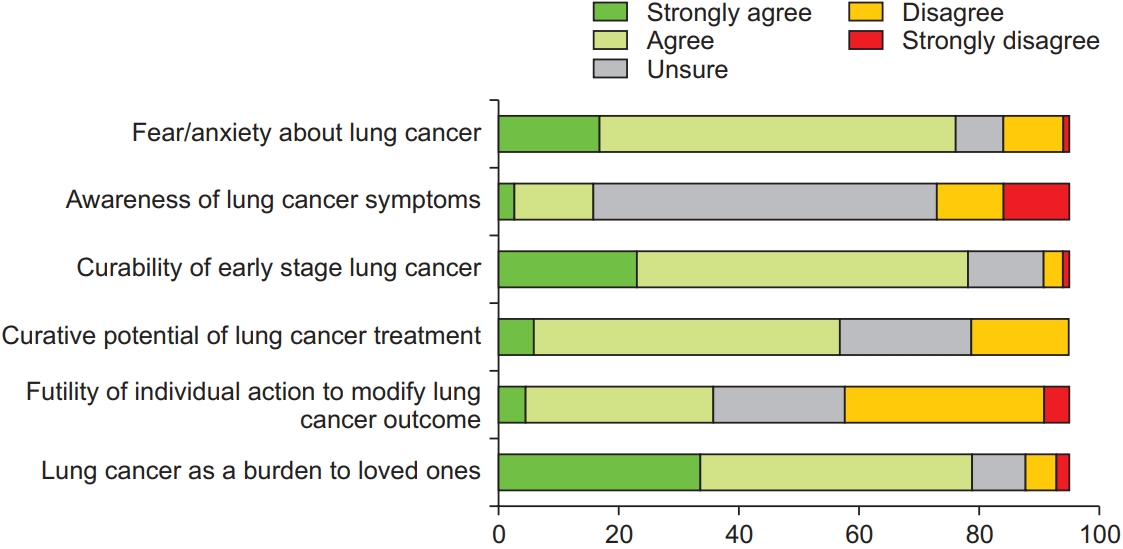
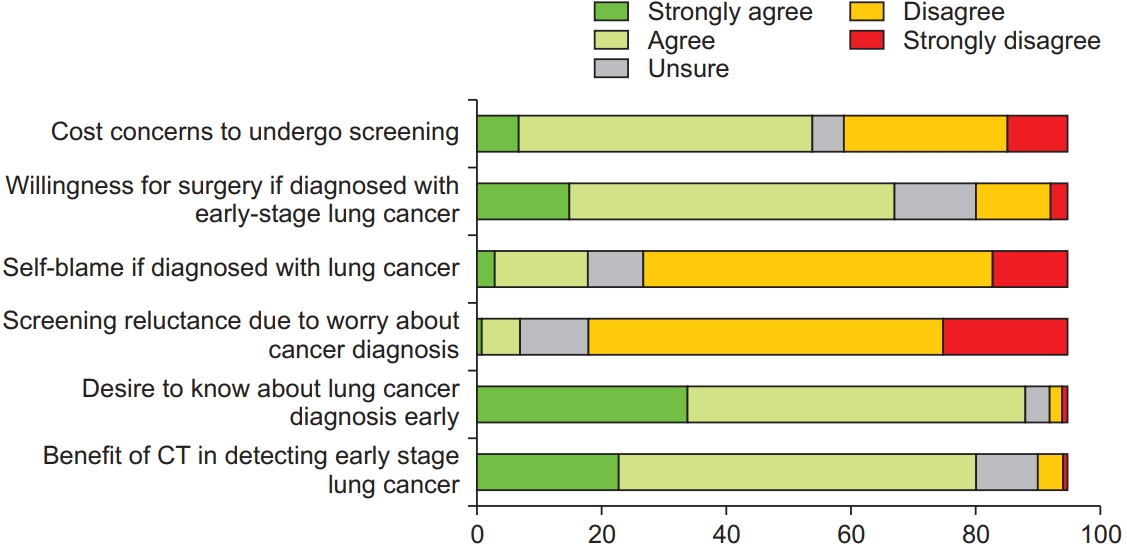
Nearly two-thirds of the respondents indicated that they were unsure about the symptoms of lung cancer; thus, demonstrating a visible knowledge gap (Figure 2, Question 2). More than three-quarters of the respondents indicated that they would experience fear and anxiety if they were diagnosed with lung cancer (Figure 2, Question 1), but preferred to know about the lung cancer diagnosis at an early-stage and agreed that LDCT was beneficial in detecting early-stage lung cancer (Figure 3, Questions 5 and 6). More than three-quarters of the respondents felt that a diagnosis of lung cancer would burden their loved ones (Figure 2, Question 6). However, most of the respondents would not blame themselves if they were diagnosed with lung cancer (Figure 3, Question 3), and one-third of the respondents feared external stigma from others (Figure 4, Question 3).
Psychosocial attitudes towards lung cancer, screening, and treatment (part two of three). CT: computed tomography.
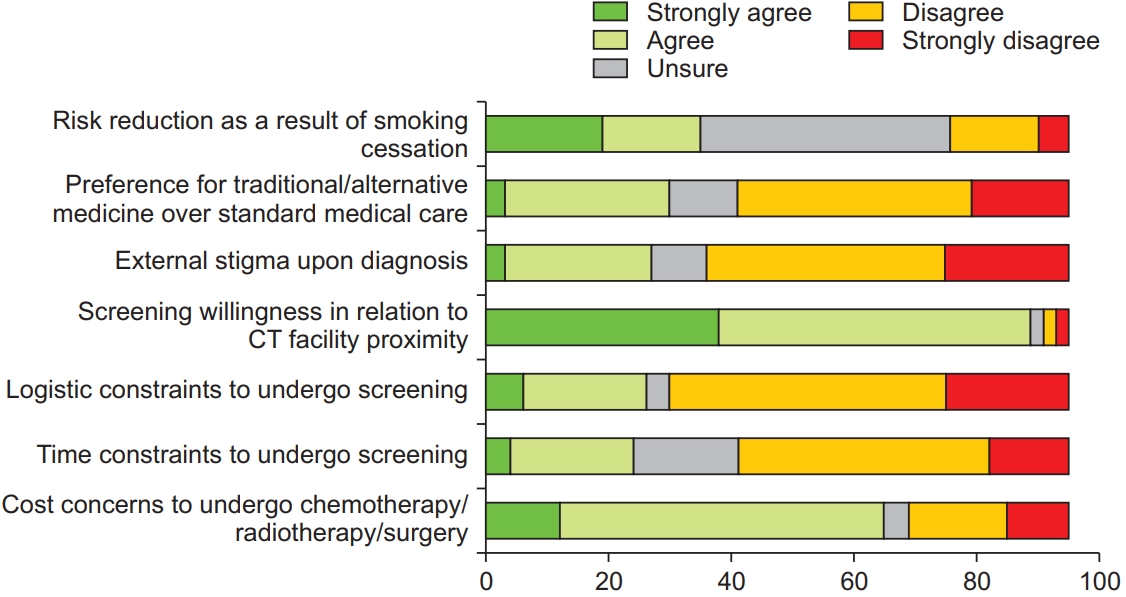
Psychosocial attitudes towards lung cancer, screening, and treatment (part three of three). CT: computed tomography.
Most of the respondents believed that early-stage lung cancer is curable, and that treatment is potentially curative (Figure 2, Questions 3 and 4), and they would be willing to undergo surgical resection if they were diagnosed with early-stage lung cancer (Figure 3, Question 2). However, one-third of the respondents would opt for traditional or alternative treatment options above standard medical care (Figure 4, Question 2). Nearly all respondents indicated increased willingness to undergo screening if the LDCT facility was located close to their homes. One-third of the respondents did not believe that smoking cessation would result in a reduction in cancer risk (Figure 4, Question 1).
A majority of the respondents were concerned about the financial implications of screening or treatment (Figure 3, Question 1; and Figure 4, Question 7). Most of the respondents disagreed that they had time or logistic constraints to undergo LDCT screening (Figure 4, Questions 5 and 6), but nearly all respondents demonstrated increasing willingness to undergo LDCT screening if the screening facility was located close to their homes.
4. Determinants of willingness to undergo screening
We used univariable binary logistic regression to examine the demographic or psychosocial factors affecting the willingness to undergo LDCT screening (Table 2). Desire to know about a lung cancer diagnosis early, having perceived time constraints to undergo screening, and having LDCT facilities closer to their place of residence were associated with significantly higher odds of willingness to undergo LDCT screening. Negative attitudes towards lung cancer treatment or ability of LDCT to detect early lung cancer, preference for traditional treatments above conventional cancer therapy, perceived burden towards loved ones, and higher education level demonstrated reduced odds to undergo screening, but they were not statistically significant.
Discussion
Despite the overwhelming willingness to undergo LDCT screening, our study highlights the potential barriers to screening uptake if a future lung cancer screening program was to be implemented. Low levels of knowledge about lung cancer symptoms, preference for traditional medication, high levels of fear and anxiety about cancer diagnosis, and high proportion of stigma among our respondents suggest a need for public advocacy and educational efforts. Low levels of knowledge may be attributed to the low educational level of our respondents. Concerns about the costs of both LDCT screening and lung cancer therapy suggest that financial implications need to be considered in a future lung screening program. Moreover, logistic factors and accessibility of screening centers are major concerns, which need to be incorporated in future lung screening programs. Community-based screening implementing mobile LDCT has been trialed with success in the United Kingdom [14]. International studies have shown that a lower socioeconomic status and current smoking status predict stigma for lung cancer, low perceived benefit, and low uptake of lung cancer screening [15,16].
Tuberculosis and COPD were the main comorbidities amongst our respondents; this reflects the burden of lung disease among our study population. While the factors of smoking initiation were not explored in this study, the relatively early median age of smoking commencement suggests a need for smoking education and intervention in educational institutions, along with a better understanding of the cultural or social drivers for smoking initiation. In terms of gender, females were likely grossly underrepresented in this study, owing to the fact that high proportion of respondents were male. Half of the current smokers were open to the possibility of stopping but had not initiated concrete plans or a stopping date; this may reflect the challenges of smoking cessation, either motivational or nicotine addiction-related challenges. Respondents who were unwilling to undergo screening consisted entirely of ex-smokers; this may have been due to a lower perceived risk, which was seen in four out of the five respondents.
The discordance in the respondents’ absolute and perceived risk suggests that individuals may sometimes underestimate their personal risk of lung cancer. It is essential that smoking cessation campaigns and educational efforts should emphasize the concept of risk among the high-risk population. However, given that a quarter of the respondents had an absolute risk score of less than 1.5%, it is unclear whether the USPSTF criteria for smoking were the most suitable criteria for our study population. Current efforts to establish screening criteria for Asians require further validation [17].
Our study is not without its limitations. Firstly, the overall sample size of 95 respondents was relatively small and consisted of inpatients; thus, it may not reflect the general population of high-risk smokers. Future, larger questionnaire studies are needed in our population. Recruitment from the private hospital study site was poor, with only two respondents being recruited. Hence, the overall findings of this study may not be generalisable to the entire population. Secondly, one limitation of using our modified version of the questionnaire was that the results may not be directly comparable to those of other studies that used other questionnaires. Third, this study did not address female non-smokers; data has shown that 60% to 80% of lung cancers in women occur in non-smokers [18]. Last but not least, environmental pollution has been linked to lung cancer, but it was not reflected in our questionnaire [19].
In conclusion, a majority of the current smokers and ex-smokers would undergo lung cancer screening if they were offered. A desire to have an early diagnosis, perceived time constraints, and proximity of LDCT screening facilities had significantly higher odds of willingness to undergo screening. Stigma, low levels of knowledge about lung cancer, financial constraints, and cultural factors were the potential barriers to lung cancer screening uptake. Lung cancer education, smoking cessation efforts, and advocacy programs need to be implemented before a regional lung cancer screening program is launched. Further research is needed to validate the findings of this pilot study.
Notes
Authors’ Contributions
Conceptualization: Nyanti LE, Ramarmuty HYD. Methodology: Nyanti LE, Ramarmuty HYD, Sivaraman Kannan KK. Formal analysis: Nyanti LE, Abd Rahim MA. Data curation: Chua CZ, Loo HC, Khor CZ, Toh ESY, Gill RS, Chan ET, Tan KY, Ibrahim A. Validation: Rosli T, Huan NC, Ramarmuty HYD. Investigation: Huan NC. Writing - original draft preparation: Nyanti LE, Abd Rahim MA. Writing - review and editing: Chua CZ, Loo HC, Khor CZ, Toh ESY, Gill RS, Chan ET, Rosli T, Ibrahim A, Huan NC, Ramarmuty HYD, Sivaraman Kannan KK. Approval of final manuscript: all authors.
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Funding
No funding to declare.
Acknowledgements
The authors would like to thank Universiti Malaysia Sabah for sponsoring the publication fees of this article.