|
 |
| Tuberc Respir Dis > Volume 87(1); 2024 > Article |
|
Abstract
Notes
AuthorsŌĆÖ Contributions
Conceptualization: Song JW. Methodology: Song JW. Formal analysis: all authors. Data curation: Lee JH. Software: all authors. Validation: Song JW. Writing - original draft preparation: Lee JH. Writing - review and editing: all authors. Approval of final manuscript: all authors.
Conflicts of Interest
Jin Woo Song is an editorial board member of the journal, but he was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest.
Funding
This study was supported by grants from the Basic Science Research Program (NRF-2022R1A2B5B0200 1602) and the Bio & Medical Technology Development Program (NRF-2022M3A9E4082647) of the National Research Foundation of Korea (NRF) funded by the Ministry of Science & ICT, Republic of Korea, as well as by grants from the National Institute of Health research project (2021ER120701) and the Korea Environment Industry & Technology Institute through the Core Technology Development Project for Environmental Diseases Prevention and Management Program funded by the Korea Ministry of the Environment (ARQ202201450001), Republic of Korea.
Figure┬Ā1.
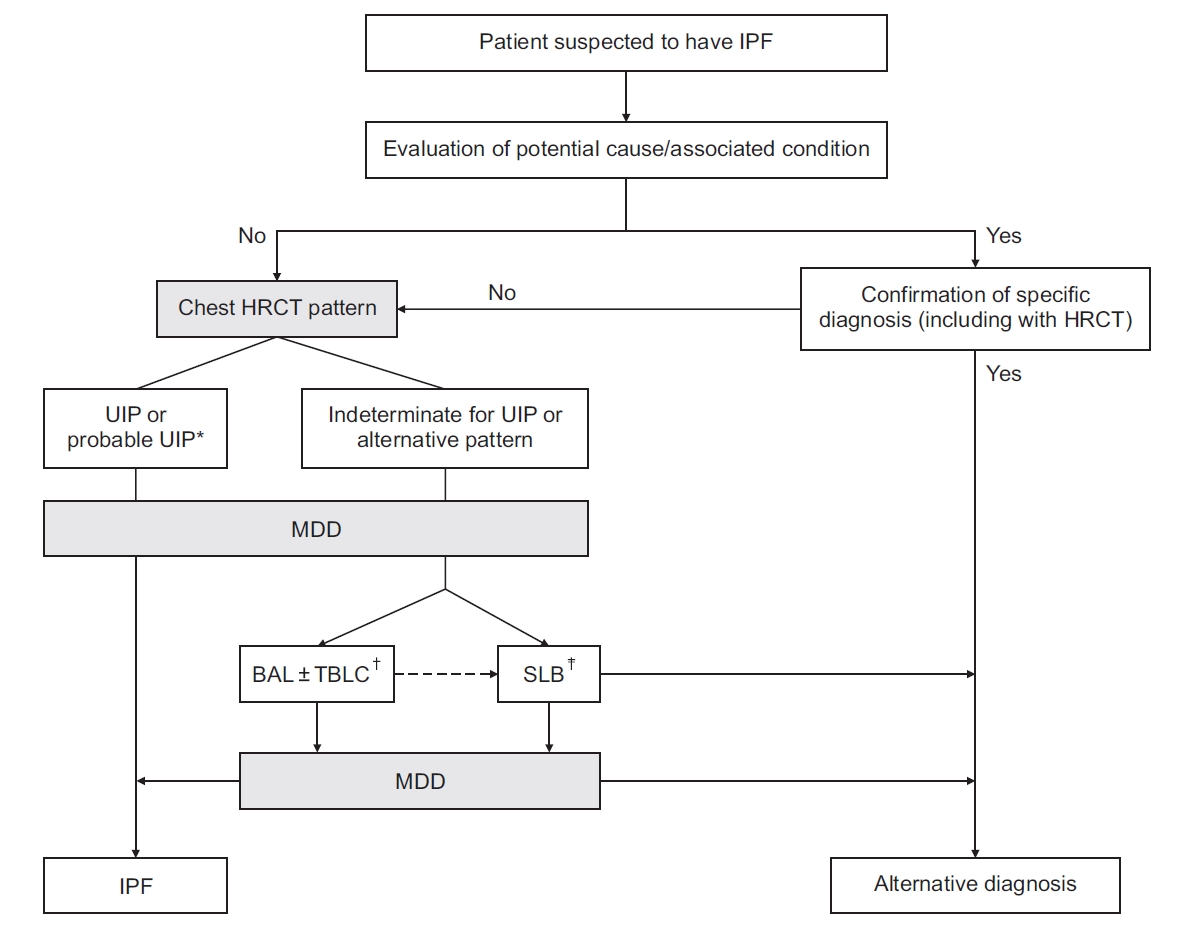
Figure┬Ā2.

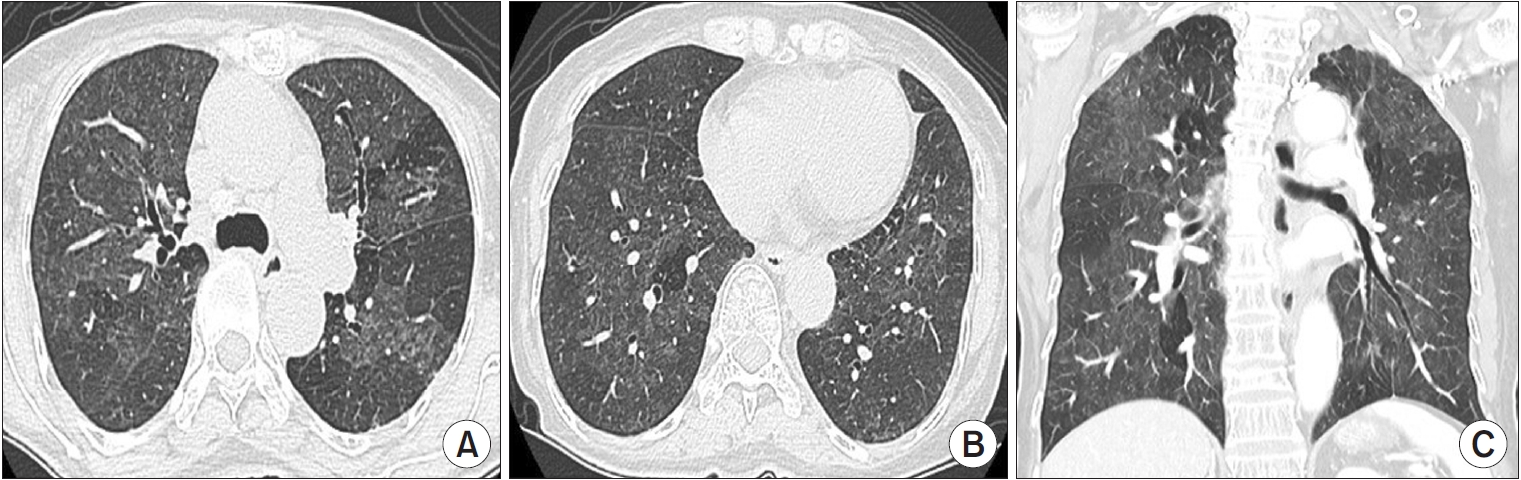
Figure┬Ā3.
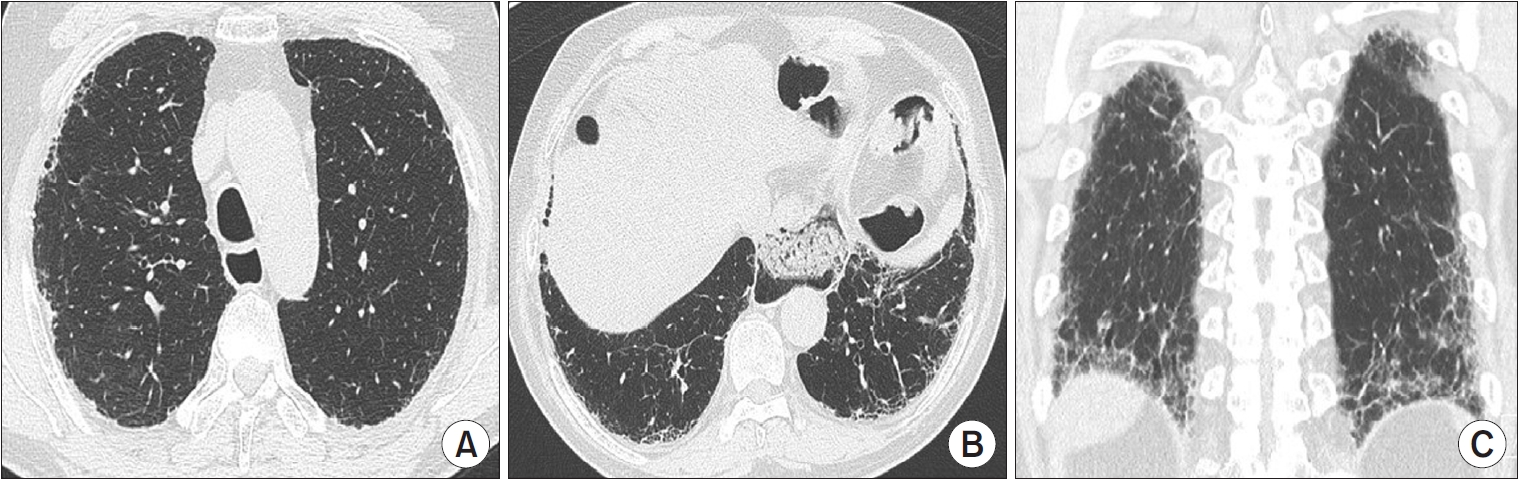
Figure┬Ā4.
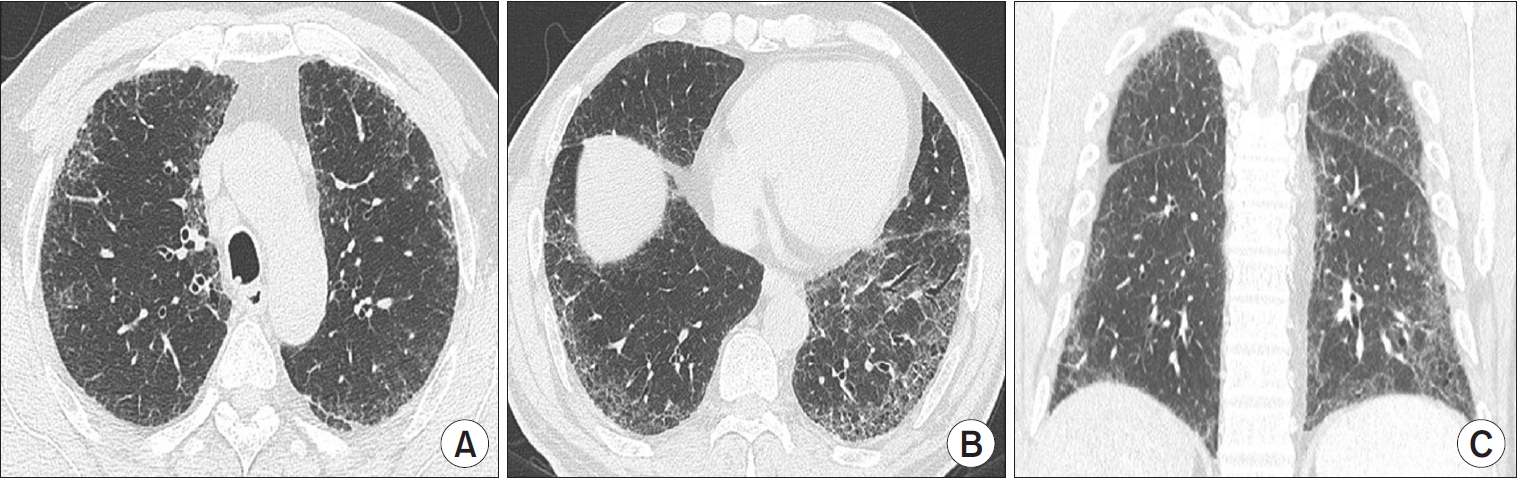
Figure┬Ā5.
Table┬Ā1.
Adopted from Raghu et al. [17]
HRCT: high-resolution computed tomography; UIP: usual interstitial pneumonia; NSIP: nonspecific interstitial pneumonia; HP: hypersensitivity pneumonitis; CTD-ILD: connective tissue disease-associated interstitial lung disease; IP: interstitial pneumonia; CT: computed tomography; GGO: ground-glass opacity; LAM: lymphangioleiomyomatosis; PLCH: pulmonary Langerhans cell histiocytosis; LIP: lymphoid interstitial pneumonia; DIP: desquamative interstitial pneumonia; CTD: connective tissue disease.
Table┬Ā2.
| UIP | Probable UIP | Indeterminate for UIP | Alternative diagnosis |
|---|---|---|---|
| Dense fibrosis with architectural distortion (i.e., destructive scarring and/or honeycombing) | Some histologic features from column 1 are present but to an extent that precludes a definite diagnosis of UIP/IPF | Fibrosis with or without architectural distortion, with features favoring either a pattern other than UIP or UIP secondary to another cause* | Features of other histologic patterns of IIPs (e.g., absence of fibroblast foci or predominant subpleural and/or loose fibrosis) in all biopsies |
| Predominant subpleural and/or paraseptal distribution of fibrosis | ŌĆāAnd | ||
| Patchy involvement of lung parenchyma by fibrosis | Absence of features to suggest an alternative diagnosis | Some histologic features from column 1 but with other features suggesting an alternative diagnosisŌĆĀ | Histologic findings indicative of other diseases (e.g., hypersensitivity pneumonitis, Langerhans cell histiocytosis, sarcoidosis, LAM) |
| Fibroblast foci | ŌĆāOr | ||
| Absence of features to suggest an alternate diagnosis | Honeycombing only |
Adopted from Raghu et al. [17]
* Granulomas, hyaline membranes (other than when associated with acute exacerbation of IPF, which may be the presenting manifestation in some patients), prominent airway-centered changes, areas of interstitial inflammation lacking associated fibrosis, marked chronic fibrous pleuritis, organizing pneumonia.
ŌĆĀ Features that should raise concerns about the likelihood of an alternative diagnosis include a cellular inflammatory infiltrate away from areas of honeycombing, prominent lymphoid hyperplasia including secondary germinal centers, and a distinctly bronchiolocentric distribution that could include extensive peribronchiolar metaplasia.
REFERENCES
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 1,634 View
- 272 Download
- ORCID iDs
-
Jae Ha Lee

https://orcid.org/0000-0003-0932-2826Jin Woo Song

https://orcid.org/0000-0001-5121-3522 - Funding Information
-
National Research Foundation of Korea
https://doi.org/10.13039/501100003725
2022R1A2B5B02001602
2022M3A9E4082647Ministry of Science and ICT
Korea National Institute of Health
https://doi.org/10.13039/501100003653
2021ER120701Ministry of Environment
https://doi.org/10.13039/501100003562
ARQ202201450001 - Related articles
-
Relapases in Pulmonary Tubersulosis
Surgical Treatment in Pulmonary Tuberculosis
Pathologic Analysis of Pulmonary Interstitial Fibrosis1990 June;37(2)
A Case of Caplan`s Syndrome with Pulmonary Tuberculosis1989 September;36(3)
Diagnostic Value of Flexible Bronchofiberscopy in Various Pulmonary Diseases1986 September;33(3)


 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Print
Print Download Citation
Download Citation