Determinants of Nicotine Dependence in Chronic Obstructive Pulmonary Disease
Article information
Abstract
Background
Smoking cessation is the most powerful intervention to modify progress of chronic obstructive pulmonary disease (COPD), and nicotine dependence is one of the most important determinants of success or failure in smoking cessation. We evaluated nicotine dependence status and investigated factors associated with moderate to high nicotine dependence in patients with COPD.
Methods
We included 53 current smokers with COPD in the Korean Obstructive Lung Disease II cohort enrolled between January 2014 and March 2016. Nicotine dependence was measured by using Fagerstrom test for nicotine dependence (FTND). Cognitive function was assessed by Korean version of Montreal Cognitive Assessment.
Results
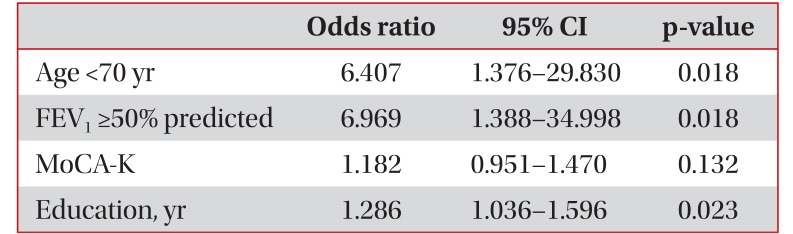
The median FTND score was 3, and 32 patients (60%) had moderate to high nicotine dependence. The median smoking amount was 44 pack-years, which was not related to nicotine dependence. Multiple logistic regression analysis revealed that high education status (odds ratio, 1.286; 95% confidence interval, 1.036–1.596; p=0.023), age <70 (odds ratio, 6.407; 95% confidence interval, 1.376–29.830; p=0.018), and mild to moderate airflow obstruction (odds ratio, 6.969; 95% confidence interval, 1.388–34.998; p=0.018) were related to moderate to high nicotine dependence.
Conclusion
Nicotine dependence does not correlate with smoking amount, but with education level, age, and severity of airflow obstruction. Physicians should provide different strategies of smoking cessation intervention for current smokers with COPD according to their education levels, age, and severity of airflow obstruction.
Introduction
Chronic obstructive pulmonary disease (COPD) is the third leading cause of death worldwide1 and affects approximately 13.4% of adults aged 40 years and over in Korea2. Cigarette smoking is the most important risk factor for development and progress of COPD3. As the amount of cigarette smoke increases, respiratory symptoms become more pronounced and lung function deteriorates3. Therefore, smoking cessation is crucial to prevent the progress of COPD134 and reduces risk of many comorbid conditions such as cardiovascular diseases and lung cancer5. Nevertheless, to stop smoking is very difficult, and smokers often fail because of nicotine addiction6. Nicotine is a psychoactive agent to regulate stress and/or to induce relaxed emotions7. The intensity of physical addiction to nicotine can be estimated by questionnaire8, which is useful for predicting the success rate of smoking cessation and provide a guide for nicotine replacement therapy9. It has been known that current smokers with COPD have higher nicotine dependence than current smokers without COPD1011. However, there were few studies on predictors of moderate to high nicotine dependence in current smokers with COPD1213.
We evaluated nicotine dependence status and investigated factors associated with moderate to high nicotine dependence in current smokers with COPD in the Korean Obstructive Lung Disease (KOLD) II cohort.
Materials and Methods
1. Patients
All subjects were selected from the KOLD cohort II, which prospectively recruited subjects with obstructive lung disease from the pulmonary clinics of 11 referral hospitals in Korea from January 2014 through March 2016. The inclusion criteria were followings: (1) age ≥40 years; (2) post-bronchodilator forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) <0.7; and (3) current smoker, who has smoked greater than 100 cigarettes in his or her lifetime and has smoked in the last 28 days. Patients with severe tuberculosis destroyed lung disease, bronchiectasis or acute lung inflammation were excluded. The study protocol was approved by the institutional review boards of the 11 hospitals.
2. Variables and measures
At the time of inclusion, a standard questionnaire was used to obtain information on smoking history, level of education, and comorbidities. COPD assessment test (CAT) was used for evaluation of COPD impact on health status. The level of dyspnea was assessed by modified Medical Research Council (MMRC) grade.
Spirometry was performed according to the American Thoracic Society/European Respiratory Society guidelines14 using the Vmax 22 (Sensor Medics, Yorba Linda, CA, USA). Postbronchodilator spirometry values were measured 15 minutes after inhalation of 400 µg of salbutamol. The spirometry reference values were based on the Korean Choi equation15.
Severe airflow obstruction is defined as FEV1 <50% predicted.
Patients responded to the Fagerstrom test for nicotine dependence (FTND)8. The FTND generates a score based on the sum of the six questionnaire items, which are variably weighted. FTND yields a total score from 0 to 10 points (mild, 0 to 3; moderate, 4 to 6; severe, 7 to 10). Subjects with a score of 4 or higher were defined as having moderate to high nicotine dependence.
Cognitive function was also assessed by Korean version of Montreal Cognitive Assessment (MoCA-K)16. The MoCA-K is a 30-point test that takes about 10 minutes to administer and consists of 12 individual tasks grouped into cognitive domains including (1) visuospatial/executive functioning, (2) naming, (3) attention, (4) language, (5) abstraction, (6) memory, and (7) orientation. A total score is calculated, and an educational correction is made (one point added for individuals with 12 years of education or less). The cognitive impairment is defined as a total score below 23.
3. Statistical analysis
Descriptive data are expressed as medians with interquartile range, and frequencies are expressed as number (%). A chi-square test was used to compare categorical variables, while continuous variables were compared using the Mann-Whitney U test. A p-value of <0.05 was regarded as statistically significant. Multiple logistic regression analysis was used to investigate factors associated with moderate to high nicotine dependence including variables, which had p-value of <0.05 in univariate analysis.
Results
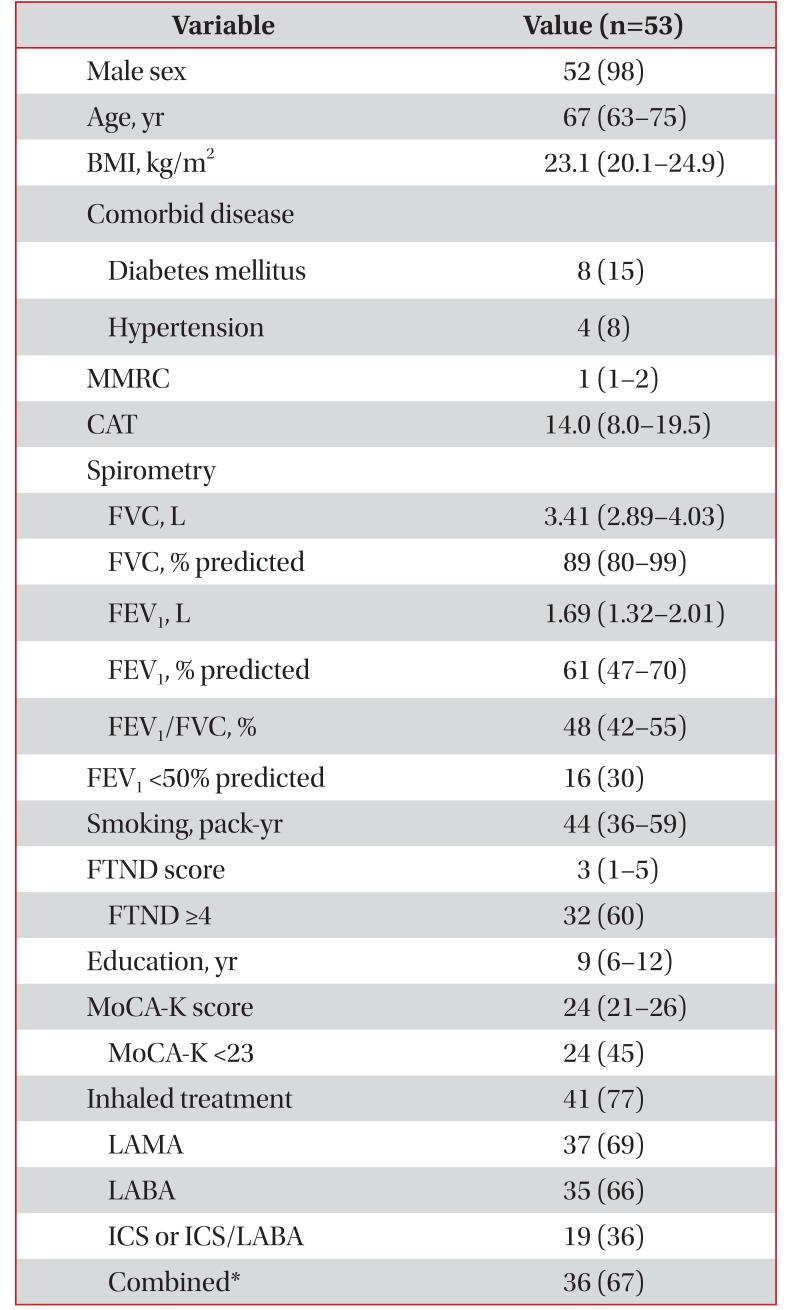
Fifty-three current smokers with COPD were included. Total 268 subjects were selected from the KOLD cohort II and excluded seven non-smokers, 204 ex-smokers, and four non-responders for questionnaire (Figure 1). Clinical characteristics of 53 patients are detailed in Table 1. Fifty-two patients (98%) were male and the median age was 67 years. The median CAT score was 14, and median MMRC dyspnea scale was 1. The median FEV1/FVC was 48% and the median FEV1 was 1.69 L (61% predicted). The patients with severe airflow obstruction were 16 (30%). The median smoking amount was 44 packyears. The median FTND score was 3, and 32 patients (60%) had moderate to high nicotine dependence. The median MoCA-K score for cognitive function was 24, and patients with cognitive dysfunction (MoCA-K <23) were 24 (45%). Fortyone patients (77%) were using inhalers.
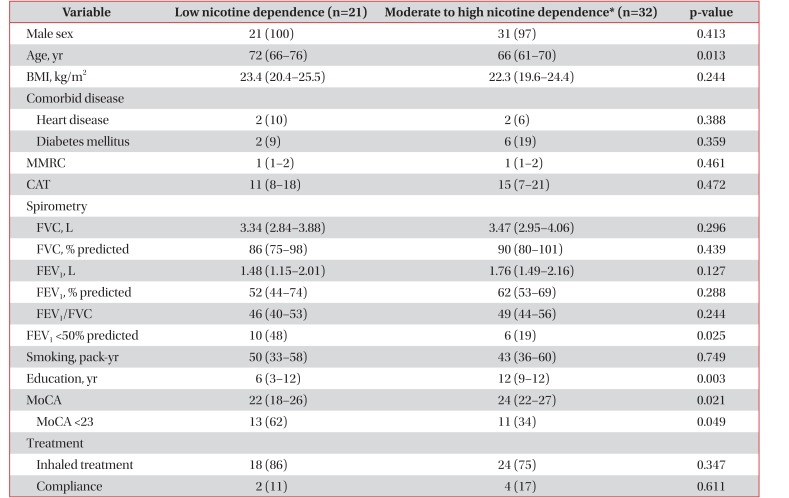
Factors related to moderate to high nicotine dependence were relatively young age (66 years vs. 76 years, p=0.013), high education status (12 years vs. 6 years, p=0.003), and high cognitive function (24 vs. 22, p=0.021). However, there are no differences of MMRC grade, CAT score, FEV1, and pack-years of cigarette smoking between two groups according to nicotine dependence (Table 2).

Factors related to moderate to high nicotine dependence of current smokers with chronic obstructive pulmonary disease
Multiple logistic regression analysis revealed that high education status (odds ratio, 1.286; 95% confidence interval, 1.036–1.596; p=0.023), age <70 (odds ratio, 6.407; 95% confidence interval, 1.376–29.830; p=0.018), and mild to moderate airflow obstruction (odds ratio, 6.969; 95% confidence interval, 1.388–34.998; p=0.018) were related to moderate to high nicotine dependence (Table 3).
Discussion
We investigated factors associated with nicotine dependence in current smokers of Korean COPD cohort. High education status was the most significant factor related to nicotine dependence in current smokers with COPD, which is consistent with a previous study17 in current smokers with diabetes. They reported that nicotine dependence in smokers who had ≥8 years of formal education was 2.57 times higher than that in those with less education. On the contrary, three previous studies showed that lower education level was linked with higher nicotine dependence among current smokers181920. These studies were different in the study subjects, one study was in patients with depressive or anxiety disorders 18, the other study was population-based19, and another study was in healthy smokers in Korea20. On the other hand, previous studies in current smokers with COPD showed that young age, high anxiety/depression, high smoking amount, low lung function, and emphysematous lung were associated with nicotine dependence1213. In a study in current smokers with COPD, education level was not related to nicotine dependence13. The disparity in the impact of education level on nicotine dependence might be due to differences in the study subjects. Even though the relationship between education levels and nicotine dependence needs to be investigated further, our study indicates that physicians should provide different strategies of smoking cessation intervention for current smokers with COPD according to their education levels. One Korean study about risk factors of high nicotine dependence in male smokers who wanted health checked up showed that nicotine dependence was related to the score of internal response, the score of external stress, the shorter duration of education, the early age of first smoking20.
In the current study, patients with moderate to high nicotine dependence showed younger age <70. It is consistent with results in previous studies: two studies included COPD patients and the other included diabetic patients121317. However, another two studies1821 showed higher nicotine dependence in older smokers: one study was population-based and the other study enrolled subjects with depressive or anxiety disorders. Based on the previous conflicting reports, relationship between nicotine dependence and age could not be exactly described. We assume that younger smokers are likely to be healthier than older smokers, which could let them not hesitate smoking cigarettes. Another reason is that working young smokers before retirement might feel more stressed and anxious, which might be linked to high nicotine dependence.
The COPD patients with better lung function showed moderate to higher nicotine dependence in this study. The study of Kim et al.12 showed similar results that the FEV1 predicted value of COPD patients with high nicotine dependence was 57.8±14.7% and the FEV1 predicted value of COPD patients with low nicotine dependence was 53.5±17.2%. The COPD patients with less respiratory symptoms had higher scores on nicotine dependence in the study of Lindberg et al.13. These results suggest that the need for smoking is higher when lung function is slightly better and respiratory symptoms are less serious.
The participants had median smoking amount of 44 packyears and median FTND of 3.0. In a study of Lindberg et al.13 of COPD patients, median smoking amount was 23.8 packyears and mean FTND score was 3.7. They showed lower median smoking amount but higher median FTND compared with those of our study. Factors associated with high FTND in their study were young age, existence of respiratory symptom, anxiety, high smoking amount, and low self efficacy13. In the other study on genetics of nicotine dependence in COPD12, mean smoking amount was 51.7 pack years and mean FTND was 4.7. Both of smoking amount and FTND were higher than those of this study. Mean age of COPD patients with high nicotine dependence was 57.9±7.5 years old and that with low nicotine dependence was 60.0±8.0 years old12. Younger patients tend to have higher nicotine dependence in our study and previous studies1213. The relatively low level of median nicotine dependence in our study might be due to the fact that many subjects were older than other studies or different characteristics of subjects including race, sex or social economic status.
Even though nicotine can enhance cognitive functions and emotional processing in some conditions22, chronic smoking is associated with a decline in cognitive functions23. There has been few study on cognitive function associated to nicotine dependence. In our study, higher cognitive function was observed in subjects with moderate to high nicotine dependence. However, nicotine dependence cannot be a favorable factor for long-term cognitive function. Since COPD patients with moderate to high nicotine dependence in this study showed younger age and higher level of education, these two factors might explain higher cognitive function in this nicotine-dependent group. To understand the relationship between cognitive function and nicotine dependence, follow-up studies on a large number of COPD patients are needed.
Although some studies of COPD smokers showed a positive correlation between smoking amount and nicotine dependence 1213, we found no difference in the amount of smoking between the low and moderate to high nicotine dependence groups. Nicotine dependence reflects current status of nicotine addiction, but not past history of smoking. In other words, it is difficult to estimate the degree of nicotine addiction through the amount of smoking in the past. Therefore, nicotine dependence measures should be made before smoking cessation education or nicotine replacement therapy rather than measuring the amount of cigarette smoking. Because current smokers of COPD exhibit moderate to high nicotine dependence, nicotine withdrawal symptoms are a major cause of failure to quit smoking, indicating that more aggressive approach for smoking cessation is needed.
The current work has some limitations. The number of participants was small. A large number of current smokers with COPD are needed to identify factors associated with nicotine dependence in COPD. It is difficult to say that our research subjects are representative of the whole COPD because they were recruited from tertiary medical institutions rather than primary medical institutions. Nevertheless, our cohort was well-designed, and surveys were conducted by well-trained nurses. Another limitation is that we did not investigate other factors known to be related to nicotine dependence such as household income, marital status, genetic factors, age at onset of smoking, depression, and drinking status.
This study assessed nicotine dependence in current smokers with COPD. We found that higher education level, mild to moderate airflow obstruction, and age <70 were associated with moderate to high nicotine dependence. In order to provide effective smoking cessation intervention for current smokers with COPD, physicians need to consider the level of individual education, age, and severity of airflow obstruction.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (2010-0027945).
Notes
Conflicts of Interest: No potential conflict of interest relevant to this article was reported.