 |
 |
| Tuberc Respir Dis > Volume 85(4); 2022 > Article |
|
Abstract
Background
Methods
Results
Notes
AuthorsŌĆÖ Contributions
Conceptualization: da Costa JC, Leite M, Greg├│rio S, Pinto JM. Methodology: da Costa JC, Manso MC, Pinto JM. Formal analysis: da Costa JC, Manso MC, Pinto JM. Data curation: da Costa JC, Manso MC. Software: da Costa JC, Manso MC. Validation: da Costa JC, Manso MC, Greg├│rio S, Leite M, Pinto JM. Investigation: da Costa JC, Manso MC, Leite M, Greg├│rio S, Pinto JM. Writing - original draft preparation: da Costa JC, Manso MC, Leite M. Writing - review and editing: da Costa JC, Manso MC, Greg├│rio S, Leite M, Pinto JM. Approval of final manuscript: all authors.
Figure┬Ā1.
Table┬Ā1.
| Variable | Total |
Death |
Univariable |
|
|---|---|---|---|---|
| Yes | No | OR (95% CI) | ||
| Sex | ||||
| ŌĆāFemale | 188 (55.3) | 35 (52.2) | 153 (56.0) | 1 |
| ŌĆāMale | 152 (44.7) | 32 (47.8) | 120 (44.0) | 1.166 (0.682-1.992) |
| Age, yr | ||||
| ŌĆāMean┬▒SD | 80.6┬▒11 | 85.8┬▒8.9 | 79.3┬▒11.1 | 1.079 (1.042-1.118) |
| ŌĆāMedian (P25-P75) | 83.5 (75.1-88) | 87.1 (82.4-91.4) | 81.6 (73-86.8) | |
| ŌĆāMin-Max | 30.7-101.9 | 51.5-99.2 | 30.7-101.9 | |
| Age, yr | ||||
| ŌĆā<86 | 197 (57.9) | 24 (35.8) | 173 (63.4) | 1 |
| ŌĆāŌēź86 | 143 (42.1) | 43 (64.2) | 100 (36.6) | 3.100 (1.776-5.409) |
| Admission time, day | ||||
| ŌĆāMean┬▒SD | 11.5┬▒7.4 | 8.9┬▒6.6 | 12.2┬▒7.4 | 0.926 (0.884-0.970) |
| ŌĆāMedian (P25-P75) | 10 (6-15) | 7 (4-12) | 11 (7-16) | |
| ŌĆāMin-Max | 1-48 | 1-28 | 1-48 | |
| ADL autonomy (Barthel index original clusters) | ||||
| ŌĆāIndependent | 60 (17.6) | 1 (1.7) | 59 (22.5) | 1 |
| ŌĆāMinimally dependent | 32 (9.4) | 2 (3.4) | 30 (11.5) | 3.933 (0.343-45.143) |
| ŌĆāPartially dependent | 38 (11.2) | 6 (10.2) | 32 (12.2) | 11.062 (1.275-95.952) |
| ŌĆāVery dependent | 41 (12.1) | 7 (11.9) | 34 (13.0) | 12.147 (1.433-102.972) |
| ŌĆāTotally dependent | 150 (44.1) | 43 (72.9) | 107 (40.8) | 23.710 (3.183-176.592) |
| ADL autonomy (Barthel index dichotomized)* | ||||
| ŌĆāIndependent | 92 (27.1) | 3 (5.1) | 89 (34.0) | 1 |
| ŌĆāDependent | 229 (67.4) | 56 (94.9) | 173 (66.0) | 9.603 (2.293-31.544) |
| CV risk factorsŌĆĀ (overall) | ||||
| ŌĆāNo | 34 (10.0) | 5 (7.5) | 29 (10.6) | 1 |
| ŌĆāYes | 306 (90.0) | 62 (92.5) | 244 (89.4) | 1.474 (0.548-3.963) |
| Hypertension | ||||
| ŌĆāNo | 103 (30.3) | 24 (35.8) | 79 (28.9) | 1 |
| ŌĆāYes | 237 (69.7) | 43 (64.2) | 194 (71.1) | 0.730 (0.415-1.282) |
| Dyslipidemia | ||||
| ŌĆāNo | 156 (45.9) | 44 (65.7) | 112 (41.0) | 1 |
| ŌĆāYes | 184 (54.1) | 23 (34.3) | 161 (59.0) | 0.364 (0.208-0.636) |
| AF | ||||
| ŌĆāNo | 278 (81.8) | 44 (65.7) | 234 (85.7) | 1 |
| ŌĆāYes | 62 (18.2) | 23 (34.3) | 39 (14.3) | 3.136 (1.708-5.759) |
| DM | ||||
| ŌĆāNo | 225 (66.2) | 41 (61.2) | 184 (67.4) | 1 |
| ŌĆāYes | 115 (33.8) | 26 (38.8) | 89 (32.6) | 1.311 (0.754-2.279) |
| Obesity | ||||
| ŌĆāNo | 272 (80.0) | 62 (92.5) | 210 (76.9) | 1 |
| ŌĆāYes | 68 (20.0) | 5 (7.5) | 63 (23.1) | 0.269 (0.104-0.698) |
| Stroke | ||||
| ŌĆāNo | 275 (80.9) | 52 (77.6) | 223 (81.7) | 1 |
| ŌĆāYes | 65 (19.1) | 15 (22.4) | 50 (18.3) | 1.287 (0.671-2.467) |
| Current stroke | ||||
| ŌĆāNo | 325 (95.6) | 65 (97.0) | 260 (95.2) | 1 |
| ŌĆāYes | 15 (4.4) | 2 (3.0) | 13 (4.8) | 0.615 (0.135-2.795) |
| CV disease | ||||
| ŌĆāNo | 241 (70.9) | 43 (64.2) | 198 (72.5) | 1 |
| ŌĆāYes | 99 (29.1) | 24 (35.8) | 75 (27.5) | 1.473 (0.837-2.594) |
| End-stage liver disease | ||||
| ŌĆāNo | 333 (97.9) | 63 (94.0) | 270 (98.9) | 1 |
| ŌĆāYes | 7 (2.1) | 4 (6.0) | 3 (1.1) | 5.714 (1.247-26.176) |
| CKD | ||||
| ŌĆāNo | 301 (88.5) | 55 (82.1) | 246 (90.1) | 1 |
| ŌĆāYes | 39 (11.5) | 12 (17.9) | 27 (9.9) | 1.988 (0.948-4.167) |
| COPD | ||||
| ŌĆāNo | 312 (91.8) | 62 (92.5) | 250 (91.6) | 1 |
| ŌĆāYes | 28 (8.2) | 5 (7.5) | 23 (8.4) | 0.877 (0.320-2.398) |
| Asthma | ||||
| ŌĆāNo | 330 (97.1) | 63 (94.0) | 267 (97.8) | 1 |
| ŌĆāYes | 10 (2.9) | 4 (6.0) | 6 (2.2) | 2.825 (0.774-10.311) |
| Interstitial lung disease | ||||
| ŌĆāNo | 337 (99.1) | 65 (97.0) | 272 (99.6) | 1 |
| ŌĆāYes | 3 (0.9) | 2 (3.0) | 1 (0.4) | 8.369 (0.747-93.712) |
| Depression | ||||
| ŌĆāNo | 298 (87.6) | 59 (88.1) | 239 (87.5) | 1 |
| ŌĆāYes | 42 (12.4) | 8 (11.9) | 34 (12.5) | 0.953 (0.419-2.167) |
| Thyroid dysfunction | ||||
| ŌĆāNo | 315 (92.6) | 65 (97.0) | 250 (91.6) | 1 |
| ŌĆāYes | 25 (7.4) | 2 (3.0) | 23 (8.4) | 0.334 (0.077-1.455) |
| Dementia | ||||
| ŌĆāNo | 264 (77.6) | 44 (65.7) | 220 (80.6) | 1 |
| ŌĆāYes | 76 (22.4) | 23 (34.3) | 53 (19.4) | 2.170 (1.207-3.902) |
| Cancer | ||||
| ŌĆāNo | 313 (92.1) | 61 (91.0) | 252 (92.3) | 1 |
| ŌĆāYes | 27 (7.9) | 6 (9.0) | 21 (7.7) | 1.180 (0.457-3.050) |
| COVID-19 symptoms | ||||
| ŌĆāNo | 47 (14.0) | 9 (13.4) | 38 (14.2) | 1 |
| ŌĆāYes | 288 (86.0) | 58 (86.6) | 230 (85.8) | 1.065 (0.487-2.326) |
| Pneumonia | ||||
| ŌĆāNo | 114 (36.2) | 13 (21.3) | 101 (39.8) | 1 |
| ŌĆāYes | 201 (63.8) | 48 (78.7) | 153 (60.2) | 2.437 (1.257-4.727) |
| Time from symptoms to diagnosis, day | ||||
| ŌĆāMean┬▒SD | 1.85┬▒5.5 | 1.02┬▒7.5 | 2.05┬▒4.9 | 0.964 (0.909-1.023) |
| ŌĆāMedian (P25-P75) | 1.0 (0-4.0) | |||
| ŌĆāMin-Max | -22 to 39 | |||
Table┬Ā2.
References
- TOOLS
-
METRICS

- ORCID iDs
-
Jo├Żo Cordeiro da Costa

https://orcid.org/0000-0003-0289-1778 - Related articles


 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link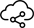 Data Sharing Statement
Data Sharing Statement Full text via DOI
Full text via DOI Print
Print Download Citation
Download Citation



