|
 |
| Tuberc Respir Dis > Volume 80(3); 2017 > Article |
|
Abstract
Background
Acute respiratory distress syndrome (ARDS) is related to high mortality and morbidity. There are no proven therapeutic measures however, to improve the clinical course of ARDS, except using low tidal volume ventilation. Metformin is known to have pleiotropic effects including anti-inflammatory activity. We hypothesized that pre-admission metformin might alter the progress of ARDS among intensive care unit (ICU) patients with diabetes mellitus (DM).
Methods
We performed a retrospective cohort study from January 1, 2005, to April 30, 2005 of patients who were admitted to the medical ICU at Seoul National University Hospital because of ARDS, and reviewed ARDS patients with DM. Metformin use was defined as prescribed within 3-month pre-admission.
Results
Of 558 patients diagnosed with ARDS, 128 (23.3%) patients had diabetes and 33 patients were treated with metformin monotherapy or in combination with other antidiabetic medications. Demographic characteristics, cause of ARDS, and comorbid conditions (except chronic kidney disease) were not different between metformin users and nonusers. Several severity indexes of ARDS were similar in both groups. The 30-day mortality was 42.42% in metformin users and 55.32% in metformin nonusers. On multivariable regression analysis, use of metformin was not significantly related to a reduced 30-day mortality (adjusted β-coefficient, −0.19; 95% confidence interval, −1.76 to 1.39; p=0.816). Propensity score-matched analyses showed similar results.
Acute respiratory distress syndrome (ARDS) is known as an acute systemic syndrome of lung inflammation characterized by increased permeability, which can result in severe hypoxia. This syndrome is of major concern for critically ill patients with increasing morbidity and mortality1. Although, inflammation is known to be involved in the pathogenesis of ARDS, several anti-inflammatory drugs have failed to improve ARDS outcomes.
The biguanide, metformin, is a widely used antidiabetic drug and recommended to newly diagnosed diabetes patients who have no contraindications2,3,4. It is well known that in addition to glucose-lowering effect and enhancement of insulin sensitivity, metformin has pleiotropic effects such as anti-inflammatory, antioxidant, endothelial barrier-enhancing, and antithrombotic effects5,6,7. Several experimental animal models of acute lung injury showed that pretreatment with metformin preserves alveolar capillary permeability; therefore, metformin decreases the occurrence and severity of acute lung injury in high-pressure ventilation8. In a recent population-based cohort study, preadmission metformin use reduced 30-day mortality among medical and surgical intensive care unit (ICU) patients with diabetes9. However, there are few studies regarding potential favorable effect of metformin focusing on patients with ARDS.
Therefore, we aimed to identify the beneficial effect of preadmission use of metformin for patients with ARDS and diabetes.
We retrospectively reviewed the medical records of patients who were admitted to the medical ICU and patients with ARDS were screened based on the International Classification of Disease 10 code at Seoul National University Hospital from January 1, 2005, to April 30, 2015. Then we confirmed adequacy of having ARDS by newly revised Berlin definition. We identified type 2 diabetes among the ARDS patients by using an algorithm incorporating any previous inpatient or outpatient records for clinical diagnosis of diabetes, any filled prescription for an antidiabetic drug or a glycated hemoglobin A1c (HbA1c) level of 6.5% or more within 3 months of the admission2.
Demographic characteristics, laboratory findings, preadmission antidiabetic drug usage, severity of illness, ventilator setting, steroid usage, interventions conducted in the ICU and clinical courses were reviewed. We excluded patients who were younger than 18 years of age clinically diagnosed as having ARDS, but who were not mechanically ventilated for various reasons, such as refusal of any invasive procedure including intubation. We also excluded patients who died within 48 hours after ICU admission.
This study was approved by the Institutional Review Board (IRB) of Seoul National University Hospital (IRB No. 1511-039-718). The requirement of informed consent from the patients was waived because of retrospective nature of the medical record review and anonymity of reporting.
For each patient, we identified all prescriptions for antidiabetic drugs within 3 months preceding admission. Prescription data were obtained from the Seoul National University Hospital electronic medical record database, or identified from medications prescribed at other hospitals. We defined metformin users as those who have taking metformin within 3 months before ICU admission; other patients with diabetes were defined as metformin nonusers.
Severity of hypoxemia was classified as mild, moderate, or severe according to the Berlin definition1. We considered other clinical factors for the severity index of ARDS including lung injury score (LIS), degree of alveolar consolidation on chest radiograph, lung compliance, time to intubation and mechanical ventilation. Both LIS and degree of alveolar consolidation on chest radiograph were assessed by same qualified clinician who has specialty in respiratory medicine.
The LIS is composed of four components: (1) chest roentgenogram score; (2) hypoxemia score; (3) positive endexpiratory pressure (PEEP) score; and (4) respiratory system compliance score, in which each component was categorized from 0 to 4 with the higher score as worse10. The total LIS was calculated by dividing the sum of each component score by the number of components used. The LIS is classified as no lung injury (0 point), mild-to-moderate lung injury (0.1-2.5 points), and severe lung injury (>2.5 points)8. For LIS, static compliance of the respiratory system is used, but it has a restricted application in retrospective study. Therefore, we supplemented dynamic lung compliance as an alternative index of ARDS severity.
We utilized a bedside chest radiograph instead of computed tomography (CT) for evaluation of alveolar consolidation because it is difficult to obtain chest CT from all patients in ICU.
In our medical ICU, we applied low tidal volume strategy (6 mL/predicted body weight) to ARDS patients and other parameters of ventilator were adjusted for each patient.
We measured clinical outcomes of ARDS patients with diabetes depending on usage of metformin including mortality, the primary outcome, and secondary outcomes such as ventilator-free days, ICU-free days and indicators of severity of ARDS.
We calculated in-hospital mortality, ventilator-free day and ICU-free day as clinical outcomes. And, we also evaluated intervention in ICU such as extracorporeal membrane oxygenation, renal replacement therapy, and tracheostomy.
Statistical tests were performed with STATA software version 13.1 (StataCorp, College Station, TX, USA). A chi-square test for comparison of categorical variables and a Student t test for continuous variables were applied. We performed univariable and thereafter multivariable logistic linear regression analysis with adjustment by confounders. A propensity score was derived from a logistic regression model used as a dichotomous dependent variable. There were few patients in the metformin use group; therefore, we performed an exact logistic regression analysis and used Firth's penalized-likelihood approach to compensate for the small sample size when we analyzed the propensity-matched cohort. We used a Kaplan-Meier curve to analyze survival. p<0.05 was considered significant.
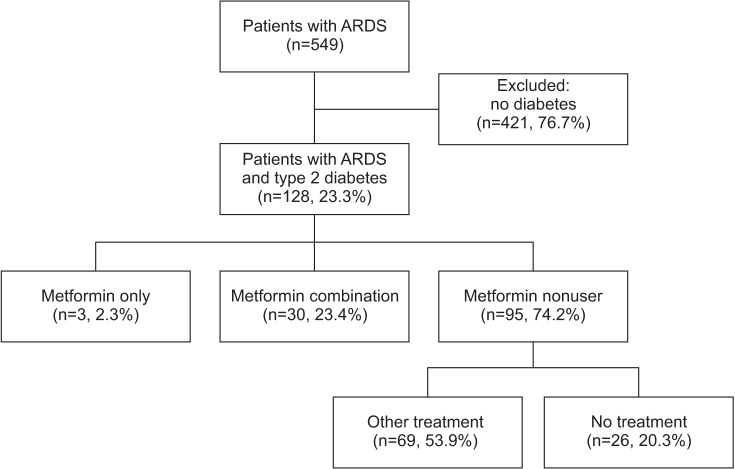
A flow diagram for the study is presented in Figure 1. Among 558 patients who met the Berlin definition of ARDS, 128 (23.3%) had diabetes. Among ARDS patients with diabetes, only three patients were treated with metformin monotherapy, 30 patients used combination treatment, and other antidiabetic medications except metformin were prescribed to 69 patients. Twenty-six patients did not use any antidiabetic medications because of favorably controlled diabetes or unawareness of diabetes until admission. Among the combination treatment group including metformin, 14 patients (46.7%) were treated with metformin and sulfonylurea, seven (23.3%) with metformin and dipeptidyl peptidase-4 inhibitor, and eight (26.7%) with more than the triple combination regimen, respectively. We divided patients into two groups: metformin users and metformin nonusers comprising patients who were treated with other antidiabetic drugs except metformin and untreated patients.
The baseline characteristics of all 128 patients who were clinically diagnosed as having ARDS with diabetes are shown in Table 1. Mean age was 69.8 years and 72.7% of metformin users were male. The mean time interval between hospital admission and ICU admission was 8.9 days for metformin users and 7.7 days for nonusers. The most common cause of ARDS was direct lung injury, such as caused by pneumonia in both groups. The comorbid conditions were not significantly different between the groups. Only numbers of patients with chronic kidney disease were significantly high among metformin nonusers.
Severity indexes such as Acute Physiology and Chronic Health Evaluation II (APACHE II) and Sequential Organ Failure Assessment (SOFA) scores were not significantly different from those for patients with or without metformin use (APACHE II: 31.8±8.7 vs. 31.0±7.6, p=0.61; SOFA: 9.9±4.2 vs. 9.6±3.5, p=0.73). The quality of blood glucose control was assessed by initial glucose level before insulin administration at the time of ICU admission and HbA1c. Although the mean initial blood glucose level was significantly higher in metformin users (251.9±98.1 vs. 210.3±89.3, p=0.03), the mean HbA1c was not different between the groups (7.3±1.0 vs. 7.3±1.4, p=0.84) (Table 2). To compare the severity of systemic inflammation, inflammatory markers including C-reactive protein, white blood cell count, and lactic acid were reviewed; however, there was no significant difference between the groups.
At the ICU admission, initial ventilator setting was not different between the groups. A large proportion of patients (>80%) had mild-to-moderate ARDS severity, and the severity was not significantly different between the groups. At the time of clinical diagnosis of ARDS, mean quadrant alveolar consolidation on chest radiographs (2.8±0.8 vs. 2.9±0.9, p=0.88) and mean LIS (2.2±0.5 vs. 2.2±0.6, p=0.95) were similar in both groups (Table 2).
Among 128 ARDS patients with diabetes mellitus, 89 patients (69.5%) died and 39 (30.5%) survived during hospital stay. Use of metformin did not significantly reduce the risk of in-hospital mortality in patients with ARDS (odds ratio [OR], 0.69; 95% confidence interval, 0.30 to 1.61; p=0.394). The 30-day mortality was higher in metformin nonusers than users, but this was not significant (Table 3, Figure 2). We also analyzed 60- and 90-day mortality, but the results were similar. At 30 days since ICU admission, mean ventilator-free days (19.9±6.5 vs. 18.4±7.2, p=0.33) and mean ICU-free days (17.1±7.1 vs. 16.1±7.5, p=0.53) were not significantly different between metformin users and nonusers. The mean total length of stay in hospital tended to be longer in nonusers (47.8±54.8 vs. 54.8±104.3, p=0.60) than users, but the difference was not significant.
The relationship between 30-day mortality and the clinical parameters was modeled using logistic regression analysis. The ORs of death within 30 days of ICU admission in unmatched and matched cohort are presented in Supplementary Table S1. In a matched cohort, body mass index, immunosuppressed patients, tachycardia, hemoglobin, lactic acid, high PEEP were significantly related to 30-day mortality. But, none of variables showed significant association with 30 days mortality in propensity matched cohort (Table 4). Neither in the unmatched cohort nor the propensity matched cohort, was there any significant association between treatment with metformin and 30-day mortality.
Despite the significantly short time between initiation of mechanical ventilation and nitric oxide (NO) use in metformin users (0.7±1.5 days vs. 8.2±7.6 days, p≤0.01), there were only a small number who used NO (10 of 30 patients vs. 30 of 95 patients, p=0.89). We also assessed the utility of venovenous extracorporeal membrane oxygenation, but only three metformin users and six nonusers were identified and there was no significant difference between them (Table 3). We also reviewed the usage of steroid during ICU care. In total, 67 patients (50.4%) were prescribed steroid after ICU admission. There is no significant difference on use of steroid between metformin users (51.4% vs. 50.0%, p=0.88) and neither in dose (734.3±391.7 mg vs. 865.7±535.3 mg, p=0.34) nor duration (21.3±3.2 days vs. 32.4±100.1 days, p=0.64) compared with metformin nonusers (data are not shown).
This study provides some support for the hypothesis that preadmission metformin could have protective effect for ARDS. We used propensity-matched analysis to overcome the weakness of our retrospective design. However, pretreatment with metformin in patients with diabetes did not show significant influence on the clinical outcome in ARDS patients.
Among 558 patients who met the Berlin definition of ARDS and were subsequently diagnosed as having ARDS, 128 (23.3%) had diabetes. This finding was consistent with the previously reported decreased incidence of ARDS in diabetic patients11. Diabetes is regarded as protective factor for the development of ARDS even after adjustment for confounders, such as age and severity of illness12. It is thought that diabetes may be involved in pathogenesis of ARDS and alter the development by altering the immune system and inflammatory response, such as adherence of neutrophils to endothelium or bactericidal activity of inflammatory cells11,13. There are several studies suggest that pretreatment with metformin attenuates ventilator-induced lung injury by preventing increased pulmonary microvascular permeability in response to deleterious mechanical ventilation in an animal model8. Also, beneficial effect of metformin on decrease in inflammatory cytokines when added to intensive insulin therapy was reported14. However, we could not identified inter-group differences of inflammatory markers (white blood cells, C-reactive protein, and lactic acid) between metformin users and nonusers and status of glycemic control by HbA1c level as well.
Most patients who clinically diagnosed as ARDS were resulted from direct lung injury and this might contributes no inter-group differences of inflammatory markers. In fact, because metformin is switched to insulin upon ICU admission and this makes any effect of preadmission metformin use in our study more vague and difficult to interpret.
Along the lines of our study, Christiansen et al.9 reported that the preadmission use of metformin was associated with a lower mortality in ICU. Because they included medical and surgical ICU patients with diabetes together, patients are more frequently continued metformin during hospitalization until the day before surgery or ICU admission. Those patients might have more chance to take their usual anti-diabetic drugs during the early phase of critical illness. On the other hand, we included only ARDS patients who admitted to medical ICU. The pleiotropic properties of metformin5,6,7 might influence the progression of ARDS, especially in the early phase. However, time interval between hospitalization and ICU admission took more than 7 days in our cohort and it implies early phase of critical illness might be went through without keeping usual anti-diabetic drugs.
Moreover, unlike the hypoglycemic effect of metformin, little is known about the anti-inflammatory effect of metformin. For example, metformin has half-life 6-18 hours on hypoglycemic effect but not in respect of anti-inflammatory effect2. There are some hypothesis for these findings, such as that metformin exerts its anti-inflammatory action by increasing formation of the endogenous nucleoside adenosine, which could potently modulate inflammation15. However, unfortunately, there are virtually no data regarding the duration and persistence of anti-inflammatory properties of metformin. It makes clinicians difficult to estimate any protective effects of preadmission metformin use on ARDS.
There were significantly more patients who had chronic kidney disease among metformin nonusers even in propensity-matched cohort, which is consistent with the strategy that metformin is seldom prescribed to patients with chronic kidney disease and shock because of concern for lactic acidosis, which is a rare adverse effect16,17. However, metformin use did not significantly affects the outcome of ARDS, even after adjustment for chronic kidney disease. Direct lung injury mainly due to the respiratory infection is frequently accompanied by acute kidney injury and about 30% of patients in both metformin users and nonusers were treated with renal replacement therapy in ICU. Progression of renal injury in infectious process before ARDS would possibly contribute discontinuation of oral hypoglycemic drugs especially metformin because of concerns over acidosis.
There are several limitations in this study. First, there was a small study population because of the single-center design. Only 33 ARDS patients with diabetes were prescribed metformin for glycemic control before ICU admission. Second, because our study is retrospective design, we could not identify the exact duration of metformin use and years follow-up period for diabetes. Third, contrary to expectations, there are few data about the anti-inflammatory pharmacokinetic action of metformin. Lastly, although we compared variety of clinical characteristics in unmatched and age, sex matched cohort, there might be confounding effects of other strategies on prognosis of patients during hospital course.
In conclusion, we did not show any significant beneficial effect of metformin on clinical outcomes of ARDS in this study. However, considering beneficial effect of metformin on the early stage of inflammation on previously reported experimental studies, further large studies are needed to evaluate the effect of pretreatment of metformin on ARDS beyond its anti-diabetic effect.
Supplementary Material
Supplementary material can be found in the journal homepage (http://www.e-trd.org).
Supplementary Table S1
Univariate logistic regression model with odds ratio for 30-day mortality (unmatched and propensity matched cohort).
References
1. ARDS Definition Task Force. Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012;307:2526-2533. PMID: 22797452.

2. American Diabetes Association. Standards of medical care in diabetes: 2013. Diabetes Care 2013;36(Suppl 1):S11-S66. PMID: 23264422.


3. Kirpichnikov D, McFarlane SI, Sowers JR. Metformin: an update. Ann Intern Med 2002;137:25-33. PMID: 12093242.


4. Nyenwe EA, Jerkins TW, Umpierrez GE, Kitabchi AE. Management of type 2 diabetes: evolving strategies for the treatment of patients with type 2 diabetes. Metabolism 2011;60:1-23. PMID: 21134520.



5. Tsoyi K, Jang HJ, Nizamutdinova IT, Kim YM, Lee YS, Kim HJ, et al. Metformin inhibits HMGB1 release in LPS-treated RAW264.7 cells and increases survival rate of endotoxaemic mice. Br J Pharmacol 2011;162:1498-1508. PMID: 21091653.



6. Grant PJ. Beneficial effects of metformin on haemostasis and vascular function in man. Diabetes Metab 2003;29(4 Pt 2):6S44-6S52. PMID: 14502100.


7. Zmijewski JW, Lorne E, Zhao X, Tsuruta Y, Sha Y, Liu G, et al. Mitochondrial respiratory complex I regulates neutrophil activation and severity of lung injury. Am J Respir Crit Care Med 2008;178:168-179. PMID: 18436790.



8. Tsaknis G, Siempos II, Kopterides P, Maniatis NA, Magkou C, Kardara M, et al. Metformin attenuates ventilator-induced lung injury. Crit Care 2012;16:R134PMID: 22827994.



9. Christiansen C, Johansen M, Christensen S, O'Brien JM, Tonnesen E, Sorensen H. Preadmission metformin use and mortality among intensive care patients with diabetes: a cohort study. Crit Care 2013;17:R192PMID: 24018017.



10. Murray JF, Matthay MA, Luce JM, Flick MR. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis 1988;138:720-723. PMID: 3202424.


11. Moss M, Guidot DM, Steinberg KP, Duhon GF, Treece P, Wolken R, et al. Diabetic patients have a decreased incidence of acute respiratory distress syndrome. Crit Care Med 2000;28:2187-2192. PMID: 10921539.


12. Hudson LD, Milberg JA, Anardi D, Maunder RJ. Clinical risks for development of the acute respiratory distress syndrome. Am J Respir Crit Care Med 1995;151(2 Pt 1):293-301. PMID: 7842182.


13. Iscimen R, Cartin-Ceba R, Yilmaz M, Khan H, Hubmayr RD, Afessa B, et al. Risk factors for the development of acute lung injury in patients with septic shock: an observational cohort study. Crit Care Med 2008;36:1518-1522. PMID: 18434908.


14. Ansari G, Mojtahedzadeh M, Kajbaf F, Najafi A, Khajavi MR, Khalili H, et al. How does blood glucose control with metformin influence intensive insulin protocols? Evidence for involvement of oxidative stress and inflammatory cytokines. Adv Ther 2008;25:681-702. PMID: 18636232.


15. Paiva M, Riksen NP, Davidson SM, Hausenloy DJ, Monteiro P, Goncalves L, et al. Metformin prevents myocardial reperfusion injury by activating the adenosine receptor. J Cardiovasc Pharmacol 2009;53:373-378. PMID: 19295441.


16. Salpeter SR, Greyber E, Pasternak GA, Salpeter EE. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database Syst Rev 2010;(4):CD002967.


17. Chan NN, Brain HP, Feher MD. Metformin-associated lactic acidosis: a rare or very rare clinical entity? Diabet Med 1999;16:273-281. PMID: 10220200.


Figure 2
The effect of preadmission metformin on 30-day mortality of acute respiratory distress syndrome patients with diabetes.
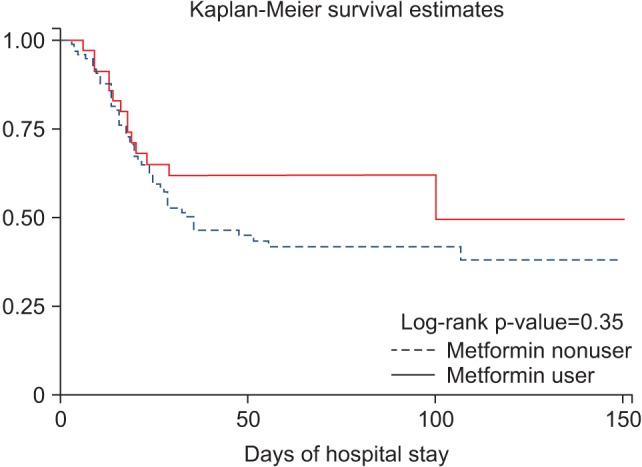
Table 1
Demographic characteristics of enrolled patients
Values are presented as mean±standard deviation or number (%) (n=128).
*Patients who has hematologic malignancy, neutropenia and taking immunosuppressant because of autoimmune disease or post-transplant graft-versus-host disease prophylaxis were defined as immunosuppressed20.
ARDS: acute respiratory distress syndrome; BMI: body mass index; ICU: intensive care unit.
Table 2
Severity of illness and respiratory parameters at the time of intensive care unit admission
Values are presented as mean±standard deviation or number (%) (n=128).
ARDS: acute respiratory distress syndrome; APACHE II score: Acute Physiology and Chronic Health Evaluation II score; SOFA score: Sequential Organ Failure Assessment score; HbA1c: glycated hemoglobin; PEEP: positive end expiratory pressure; PIP: peak inspiratory pressure; FiO2: fraction of inspired oxygen.


 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Supplement
Supplement Print
Print Download Citation
Download Citation