 |
 |
| Tuberc Respir Dis > Volume 80(2); 2017 > Article |
|
Abstract
Background
Methicillin-resistant Staphylococcus aureus (MRSA) infection is a severe and life-threatening disease in patients with community-onset (CO) pneumonia. However, the current guidelines lack specificity for a screening test for MRSA infection.
Methods
This study was retrospectively conducted in elderly patients aged ≥65 years, who had contracted CO-pneumonia during hospitalization at the Jeju National University Hospital, between January 2012 and December 2014. We analyzed the risk factors of MRSA in these patients and developed a scoring system to predict MRSA infection.
Results
A total of 762 patients were enrolled in this study, including 19 (2.4%) with MRSA infection. Healthcare-associated pneumonia (HCAP) showed more frequent MRSA infection compared to community-acquired pneumonia (4.4% vs. 1.5%, respectively; p=0.016). In a multivariate logistic regression analysis, admissions during the influenza season (odds ratio [OR], 2.896; 95% confidence interval [CI], 1.022-8.202; p=0.045), chronic kidney disease (OR, 3.555; 95% CI, 1.157-10.926; p=0.027), and intensive care unit admission (OR, 3.385; 95% CI, 1.035-11.075; p=0.044) were identified as predictive factors for MRSA infection. However, the presence of HCAP was not significantly associated with MRSA infection (OR, 1.991; 95% CI, 0.720-5.505; p=0.185). The scoring system consisted of three variables based on the multivariate analysis, and showed moderately accurate diagnostic prediction (area under curve, 0.790; 95% CI, 0.680-0.899; p<0.001).
Conclusion
MRSA infection would be considered in elderly CO-pneumonia patients, with three risk factors identified herein. When managing elderly patients with pneumonia, clinicians might keep in mind that these risk factors are associated with MRSA infection, which may help in selecting appropriate antibiotics.
Among causative organisms of community-acquired pneumonia (CAP), Staphylococcus aureus accounts for about 1% to 5% of all CAP infections1. In addition, methicillin-resistant Staphylococcus aureus (MRSA) has been generally regarded as a major pathogen of hospital-acquired pneumonia (HAP) and ventilator-associated pneumonia (VAP)2. However, the incidence of MRSA infection has increased worldwide among community-acquired (CA) as well as hospital-acquired pathogens3. Although CA-MRSA pneumonia is rare, it has been associated historically with influenza outbreaks and multi-lobar cavitating necrosis1.
As MRSA infection presents with significant morbidity and mortality in patients with community-onset (CO) pneumonia including CAP and healthcare-acquired pneumonia (HCAP)4, it is important to predict the occurrence of MRSA infection in these patients. Recently, the 2016 the American Thoracic Society/Infectious Disease Society of America (ATS/IDSA) guidelines for management of HAP and VAP suggested that risk factors for MRSA infection included prior intravenous antibiotic use within 90 days, hospitalization in a unit where >20% of Staphylococcus aureus isolates are methicillin resistant, or the prevalence of MRSA is not known, or who are at high risk for mortality5. However, the determination for empirical treatment with anti-MRSA agents has not yet been established in patients with CO-pneumonia6. According to the 2007 ATS/IDSA guidelines for CAP, necrotizing or lung abscess is a risk factor for CA-MRSA pneumonia6. Although a recently published study in the United States investigated the use of a scoring system to predict MRSA infection in patients with CO-pneumonia, data on the prediction of MRSA infection in these patients are still limited7.
MRSA infection requires treatment with anti-MRSA agents that are distinct from empirical antibiotic treatments used for CO-pneumonia. Delayed use of anti-MRSA agents may result in increased morbidity and mortality, whereas overuse of anti-MRSA agents may lead to more antibiotic resistance and side effects associated with antibiotic use such as nephrotoxicity. In the elderly population, pneumonia is especially associated with worse outcomes8,9. In addition, the large number of elderly with decreased renal function requires careful use of anti-MRSA agents in this population. Therefore, we investigated the risk factors for MRSA infection in elderly patients admitted with CO-pneumonia in order to guide the decision to prescribe an anti-MRSA agent.
We retrospectively investigated elderly patients aged ≥65 years admitted with CO-pneumonia at the Jeju National University Hospital (a 620-bed hospital in Jeju, South Korea) between January 2012 and December 2014. Based on culture results, we classified patients into MRSA or non-MRSA groups.
Using medical records, we compared clinical characteristics, co-morbidities, severity of pneumonia, identified pathogens, antibiotics, and clinical outcomes between the two groups. The study protocol was approved by the Ethical Review Committee at Jeju National University Hospital (approval No. 2015-03-002). Informed consent was waived because of the retrospective nature of the study.
Pneumonia was defined as the presence of new infiltrate on chest radiography and at least one of the following: fever (temperature ≥38.0℃) or hypothermia (temperature <35.0℃); new-onset cough with or without sputum production; pleuritic chest pain; dyspnea; or altered breath sounds on auscultation. Multi-lobar involvement was defined as the presence of pneumonic infiltrates in two or more lobes on chest radiograph or computed tomography.
HCAP was defined using the criteria established by the 2005 ATS/IDSA guidelines, which require one or more of the following: residence in a nursing home or long-term care facility; recent history of hospitalization in an acute care hospital for ≥2 days in the past 90 days; recent outpatient intravenous therapy (such as antibiotic therapy or chemotherapy) or wound care within the past 30 days; and/or attendance at a hospital clinic or dialysis center in the last 30 days10. CAP was defined as pneumonia diagnosed in patients who did not fit the criteria for HCAP. CO-pneumonia was defined as pneumonia hat occurs in the community and up to 48 hours into hospital admission, and it includes both CAP and HCAP11.
Microorganisms in samples obtained from sputum, tracheal aspirate, bronchial alveolar lavage fluid, or blood were investigated. Sputum samples were cultured in a semi-quantitative manner, and pathogens were identified when a predominant microorganism was detected from group 4 or 5 sputum, according to Geckler's grading system12. Blood cultures were considered as an etiologic diagnosis if there was no other infection source for a positive blood culture. For Mycoplasma pneumoniae or Chlamydia pneumoniae, serum samples were evaluated. Serum samples in which particle agglutination antibody titers were >64 or that were proven to have a 4-fold or greater increase of antibody titers in paired sera were regarded as positive. BinaxNOW (Binax Inc., Scarborough, ME, USA) was used to detect urinary antigens for Streptococcus pneumoniae. The BinaxNOW Legionella Urinary Antigen Test (Binax Inc.) for Legionella pneumophila serogroup 1 was performed according to clinical judgment by attending physicians. A positive urinary antigen was considered to be a bacterial infection. The antibiotic sensitivity of all isolates was determined using a disc diffusion method. MRSA, Pseudomonas species, Acinetobacter species, Stenotrophomonas maltophilia, and extended-spectrum β-lactamase (ESBL) producing Enterobacteriaceae were considered to be potentially drug resistant (PDR) pathogens, as previously reported13.
Duration of antibiotic therapy, the rate of change of antibiotics, the use of inappropriate antibiotics, the rate of failure of initial antibiotic therapy, length of hospital stay, and inhospital mortality rates were compared between each group. Inappropriate antibiotic therapy was defined as inefficacy of the empirical antibiotics against the identified pathogens based on in vitro susceptibility testing. Failure of initial antibiotic therapy was defined as death during initial treatment or change of antibiotics from initial agents to others after 48 hours due to clinical instability.
Data are presented as number (%) or median (range) unless otherwise stated. Continuous variables were compared using Student's t test for normally distributed variables and the Mann-Whitney U test for non-normally distributed variables. Categorical variables were compared using the Pearson chi-square test, and the Fisher exact test was used where any cell contained less than 5. To identify independent factors predictive of MRSA infection, multivariate logistic regression analysis with variables found to be significantly different between the two groups by univariate analysis was used, as measured by the estimated odds ratio (OR) with 95% confidence intervals (CI). From logistic regression results, we created a scoring system to predict patients' risk for infection with MRSA pathogens. We converted the ORs from β coefficients into point values and measured the percentages of MRSA pathogens in each group relative to the total point score. We then evaluated the ability of the scoring system to predict patients with MRSA pathogens using the receiver operating characteristic (ROC) curve. p-values of <0.05 were considered statistically significant. Analyses were performed using SPSS version 14.0 (SPSS Inc., Chicago, IL, USA).
A total of 762 patients were assessed during the study period. According to culture results, there were 19 patients (2.4%) with MRSA infection and 743 (97.5%) with non-MRSA infection. MRSA infection was more common in HCAP than CAP (4.4% vs. 1.5%, p=0.016).
Table 1 shows the baseline characteristics of each group. The median age of the MRSA group was 81 years (interquartile range [IQR], 78-84 years) and was higher than that of the non-MRSA group (75 years; IQR, 68-81 years; p=0.004). The rate of patients with HCAP was higher in the MRSA group compared to the non-MRSA group (57.8% vs. 31.7%, p=0.016). Among the category of HCAP, the rate of residence in a nursing home or long-term care facility was more frequently in the MRSA group (36.8% vs. 15.3%, p=0.020). And, the rate of patients with aspiration tendency tended to be higher in the MRSA group than the non-MRSA group (47.3% vs. 27.8%, p=0.062). Among several comorbidities, only chronic kidney disease was frequently more reported in the MRSA group (31.5% vs. 11.3%, p=0.017).
The MRSA group showed worse clinical parameters than the non-MRSA group, including altered mental state, sepsis or septic shock, and intensive care unit (ICU) admission at onset. In radiological findings, the rates of multi-lobar involvement and pleural effusions between the two groups were not significantly different. The median CURB-65 (confusion, urea, eespiratory rate, blood pressure, age ≥65) and Pneumonia Severity Index (PSI) scores, which are severity indices of pneumonia, were also higher in the MRSA group (3 vs. 2, p<0.001 and 146 vs. 104, p<0.001).
MRSA was isolated from the specimens of the followings: sputum (n=13), tracheal aspirates (n=4), blood (n=3), and bronchoalveolar lavage fluid (n=1). In two patients, MRSA were simultaneously isolated from the tracheal aspirates and blood.
The distribution of pathogens isolated in the non-MRSA group is shown in Table 2. In the non-MRSA group, there was a possible etiologic diagnosis for 271 patients (36.4%); the most frequent pathogen was Streptococcus pneumoniae followed by Klebsiella pneumoniae. The frequency of PDR pathogens was 7.8%, and the isolated rates of Pseudomonas aeruginosa, ESBL-producing K. pneumoniae, and Acinetobacter species were 5.2%, 0.4%, and 1.2%, respectively.
Table 3 shows the clinical outcomes of patients with pneumonia. The duration of anti-biotic administration (14 days vs. 10 days, p=0.027), the rates of antibiotic changes (73.6% vs. 20.8%, p<0.001), the use of inappropriate antibiotics (100% vs. 4.7%, p<0.001), and failure of initial antibiotic therapy (73.6% vs. 20.5%, p<0.001) were higher in the MRSA group. In addition, the MRSA group showed a trend toward a higher total inhospital mortality rate than the non-MRSA group, although without statistical significance (31.2% vs. 11.4%, p=0.062).
Multivariate logistic regression analysis identified three risk factors related to MRSA pneumonia. Admission during influenza season (OR, 2.896; 95% CI, 1.022-8.202; p=0.045), chronic kidney disease (OR, 3.555; 95% CI, 1.157-10.926; p=0.027), and ICU admission (OR, 3.385; 95% CI, 1.035-11.075; p=0.044) were associated with increased risk for MRSA infection in elderly patients admitted with CO-pneumonia. Otherwise, the presence of HCAP was not significantly associated with MRSA infection (OR, 1.991; 95% CI, 0.720-5.505; p=0.185) (Table 4).
Based on the multivariate analysis, we created a scoring system to identify patients with MRSA infection. We assigned points as follows based on the logistic regression: 2 points for admission during influenza season; 3 points for chronic kidney disease; 3 points for ICU admission. Patients were divided into three groups by total scores. Figure 1 shows the association between total score using the scoring system and the prevalence of MRSA infection. As scores increased, the prevalence of MRSA pathogen in each group tended to increase (0.5% in the group with a score of 0, 2.2% in the group with a score 2-3, and 13.0% in the group with scores 5-8). Using ROC curves, the area under curve of the scoring system was 0.7900 (95% CI, 0.6801-0.8998; p<0.001) (Figure 2).
MRSA infections are associated with worse outcomes than methicillin-sensitive Staphylococcus aureus infections14. One meta-analysis including data on 3,963 patients reported that bloodstream infection due to MRSA showed approximately a twofold increase in mortality compared to patients with methicillin-sensitive Staphylococcus aureus14. Traditionally, MRSA infection is common cause of HAP and VAP15. Meanwhile, MRSA infection in patients with CAP is rare, and CA-MRSA pneumonia represents about 2% of total CA-MRSA infections16. Although the incidence of MRSA infection is low in patients with CO-pneumonia, MRSA infection is an emerging problem in these patients4.
The increased prevalence of MRSA infection in CAP has been reported in patients with recent or concomitant influenza17,18. During the 2003 to 2004 influenza season in the United States, 17 patients with Staphylococcus aureus infection in CAP were reported and 15 cases were CA-MRSA17. All isolates had community-associated genetic characteristics. Among 13 available Staphylococcus aureus isolates, 12 had the Panton-Valentine leukocidin (PVL) gene, and in-hospital mortality was 29%17. In a study of 627 patients admitted with CAP during the 2006 to 2007 influenza season in the United States, Staphylococcus aureus represented about 4% (24 patients) of causative organisms. Of these Staphylococcus aureus infections, 14 cases (58%) were MRSA19. Isolation of MRSA was associated with the followings; a patient history of MRSA, nursing home admission in the previous year, close contact with someone with a skin infection during the previous month, multiple infiltrates or cavities on a chest radiograph, comatose state, intubation, receipt of pressor agents, and death in the emergency department19. Although we could not evaluate the direct relationship between MRSA infection and influenza due to the low frequency of testing for influenza infection, patients who were hospitalized during the influenza season (December to March) in the present study showed a significantly higher rate of isolation for MRSA.
Traditionally, CA-MRSA is also associated with severe necrotizing pneumonia3,17,20. The tendency for necrotizing pneumonia seems to be mediated by PVL, which is typically present in CA-MRSA strains17,20,21. Gillet et al.21 compared the clinical features of 16 patients with PVL-positive Staphylococcus aureus pneumonia to 36 cases of PVL-negative Staphylococcus aureus pneumonia20. Hemoptysis occurred more frequently in patients with PVL-positive strains compared with those with PVL-negative strains (38% vs. 3%)21. The role of PVL was more directly demonstrated in a study using a mouse model of acute pneumonia, which found that PVL alone was sufficient to cause necrotizing pneumonia22. PVL induced global changes in the transcriptional levels of genes encoding multiple staphylococcal proteins, including the lung inflammatory factor staphylococcal protein A22. In contrast, some recent reports have denied the role of the PVL gene as a virulence factor in MRSA pneumonia23,24. In our results, the radiological findings were not significant differences between two groups. Otherwise, due to nature of retrospective study design, we could not investigate the association between the MRSA infection and necrotizing pneumonia.
After the ATS/IDSA introduced guidelines in 2005 on HCAP10, several studies have reported an association between HCAP and an increased risk of MRSA infection4,11,25. Therefore, the guidelines recommended that HCAP patients should receive PDR-targeted antibiotic treatment, including anti-MRSA agents and/or anti-pseudomonal agents, similar to HAP patients10. A recent meta-analysis compared the isolated rates of pathogens between patients with HCAP and CAP26. MRSA was identified in 9.7% of patients (624/6,368) with HCAP and 2.4% of patients (280/11,302) with CAP26. And, HCAP was associated with an increased risk of MRSA infection (OR, 4.72; 95% CI, 3.69-6.04; p<0.0001)26. However, several recent studies on HCAP showed poor adherence to the 2005 ATS/IDSA antibiotic guidelines, and controversy has increased regarding the appropriate treatment for HCAP compared to CAP and HAP27. In addition, studies testing the prediction of MRSA infection and optimal selection of anti-MRSA agents in patients with HCAP were limited4,27,28. Moreover, the 2005 ATS/IDSA antibiotic guidelines for HCAP did not establish a clear indication to select initial anti-MRSA agents10. In our study, although MRSA infection was more frequent in patients with HCAP compared to CAP (4.4% vs. 1.5%, p=0.016), the presence of HCAP itself was not an independent, predictive factor for MRSA infection. But, because this study included small sample of the MRSA group, we could not draw concrete conclusions.
In 2013, the U.S. large cohort study introduced a new MRSA prediction score in patients with CO-pneumonia7. This scoring system was based on 5,975 patients with CO-pneumonia from 62 U.S. hospitals, and MRSA infections were detected in 837 patients (14.0%). The risk score consisted of eight variables: two for recent hospitalization or ICU admission and one each for age <30 or >79 years, prior intravenous antibiotic exposure, dementia, cerebrovascular disease, female with diabetes, or nursing home-acquired pneumonia7. The total score was 107. When the score was low (0 or 1), the prevalence of MRSA was <10%, whereas when the score was high (6 or greater), the prevalence of MRSA was predicted as >30%7. A subsequent retrospective study compared the effect of anti-MRSA agents on 30-day patient mortality among CO-pneumonia patients in three MRSA risk groups (low, medium, and high-risk)11. In the high-risk group, initial anti-MRSA treatment was associated with a lower 30-day mortality (OR, 0.57; 95% CI, 0.42-0.77)11.
Recently, one retrospective observational study on risk factors associated with MRSA infections has been conducted in CO-pneumonia Korean patients4. In this study, MRSA infection was detected in 8.2% (78/943 patients), and the MRSA group showed higher mortality than the non-MRSA group (33.3% vs. 21.5%, p=0.017)4. Risk factors for MRSA infection included a history of MRSA infection in the previous 1 year (OR, 6.05; 95% CI, 2.99-12.22; p<0.001), PSI score higher than 120 (OR, 2.40; 95% CI, 1.18-4.86; p=0.015), and intravenous antibiotic treatment within 30 days (OR, 2.23; 95% CI, 1.15-4.32; p=0.018)4. In this study, the incidence of the MRSA infections was 12.7% in patients with HCAP and 4.2% in those with CAP, respectively. Due to the higher incidence of MRSA infections than in previous studies26, these findings might not be generally applicable to other Korean hospitals.
Based on our knowledge, the present study is the first to investigate MRSA infection in elderly patients aged ≥65 years with CO-pneumonia in Korea. But, the present study has some limitations. First, the present study was retrospectively conducted at a single center. Therefore, physicians should consider interregional differences in microbiology. Second, we could not collect certain data on the MRSA group. And, this study examined relatively few patients of the MRSA group, and studies looking at a larger group of patients are needed in the future. Also, in the present study, relatively low numbers of non-MRSA pneumonia patients had causative microorganisms (271 of 743 non-MRSA patients, 36.4%). To evaluate the risk factor accurately, more sufficient numbers of non-MRSA patients with proved microorganisms might have been included in the analysis. Third, when MRSA was identified in the culture, patients were classified as the MRSA group. However, some identified micro-organisms may have been oropharyngeal colonizers or contaminants and may not have been the definite cause of pneumonia. Lastly, according to the recent population-based surveillance study, respiratory viruses were detected more frequently than bacteria in the CAP patients. Viruses were detected in 27% of these patients and the most commonly isolated virus were human rhinovirus (in 9% of patients), influenza virus (in 6%)29. But, because we did not perform viral testing in most of patients, we could not evaluate a role for virus in the MRSA pneumonia.
In conclusion, although the present study showed a low incidence (2.4%) of MRSA infection in elderly patients admitted with CO-pneumonia, and MRSA infection was more common in HCAP than CAP. MRSA infection was associated with worse outcomes in these patients. Results from this study suggested that predictive factors for MRSA infection included admission during influenza season, chronic kidney disease, and ICU admission. However, the presence of HCAP was not determined to be risk factors for MRSA infection. The scoring system using three variables showed moderately accurate diagnostic accuracy. Further large scale studies are needed to identify predictive factors for MRSA infection in these patients.
References
1. Kwong JC, Chua K, Charles PG. Managing severe community-acquired pneumonia due to community methicillin-resistant Staphylococcus aureus (MRSA). Curr Infect Dis Rep 2012;14:330-338. PMID: 22430229.



2. Rubinstein E, Kollef MH, Nathwani D. Pneumonia caused by methicillin-resistant Staphylococcus aureus. Clin Infect Dis 2008;46(Suppl 5):S378-S385. PMID: 18462093.



3. Lobo LJ, Reed KD, Wunderink RG. Expanded clinical presentation of community-acquired methicillin-resistant Staphylococcus aureus pneumonia. Chest 2010;138:130-136. PMID: 20173050.


4. Jung WJ, Kang YA, Park MS, Park SC, Leem AY, Kim EY, et al. Prediction of methicillin-resistant Staphylococcus aureus in patients with non-nosocomial pneumonia. BMC Infect Dis 2013;13:370PMID: 23937553.



5. Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 2016;63:e61-e111. PMID: 27418577.




6. Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007;44(Suppl 2):S27-S72. PMID: 17278083.




7. Shorr AF, Myers DE, Huang DB, Nathanson BH, Emons MF, Kollef MH. A risk score for identifying methicillin-resistant Staphylococcus aureus in patients presenting to the hospital with pneumonia. BMC Infect Dis 2013;13:268PMID: 23742753.



8. Fein AM, Niederman MS. Severe pneumonia in the elderly. Clin Geriatr Med 1994;10:121-143. PMID: 8168019.


9. Li W, Ding C, Yin S. Severe pneumonia in the elderly: a multivariate analysis of risk factors. Int J Clin Exp Med 2015;8:12463-12475. PMID: 26550157.


10. American Thoracic Society. Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005;171:388-416. PMID: 15699079.


11. Teshome BF, Lee GC, Reveles KR, Attridge RT, Koeller J, Wang CP, et al. Application of a methicillin-resistant Staphylococcus aureus risk score for community-onset pneumonia patients and outcomes with initial treatment. BMC Infect Dis 2015;15:380PMID: 26385225.



12. Geckler RW, Gremillion DH, McAllister CK, Ellenbogen C. Microscopic and bacteriological comparison of paired sputa and transtracheal aspirates. J Clin Microbiol 1977;6:396-399. PMID: 334796.



13. Trouillet JL, Chastre J, Vuagnat A, Joly-Guillou ML, Combaux D, Dombret MC, et al. Ventilator-associated pneumonia caused by potentially drug-resistant bacteria. Am J Respir Crit Care Med 1998;157:531-539. PMID: 9476869.


14. Cosgrove SE, Sakoulas G, Perencevich EN, Schwaber MJ, Karchmer AW, Carmeli Y. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin Infect Dis 2003;36:53-59. PMID: 12491202.



15. Bartlett JG, Mundy LM. Community-acquired pneumonia. N Engl J Med 1995;333:1618-1624. PMID: 7477199.


16. Fridkin SK, Hageman JC, Morrison M, Sanza LT, Como-Sabetti K, Jernigan JA, et al. Methicillin-resistant Staphylococcus aureus disease in three communities. N Engl J Med 2005;352:1436-1444. PMID: 15814879.


17. Hageman JC, Uyeki TM, Francis JS, Jernigan DB, Wheeler JG, Bridges CB, et al. Severe community-acquired pneumonia due to Staphylococcus aureus, 2003-04 influenza season. Emerg Infect Dis 2006;12:894-899. PMID: 16707043.



18. Kallen AJ, Brunkard J, Moore Z, Budge P, Arnold KE, Fosheim G, et al. Staphylococcus aureus community-acquired pneumonia during the 2006 to 2007 influenza season. Ann Emerg Med 2009;53:358-365. PMID: 18534715.


19. Moran GJ, Krishnadasan A, Gorwitz RJ, Fosheim GE, Albrecht V, Limbago B, et al. Prevalence of methicillin-resistant Staphylococcus aureus as an etiology of community-acquired pneumonia. Clin Infect Dis 2012;54:1126-1133. PMID: 22438343.



20. Francis JS, Doherty MC, Lopatin U, Johnston CP, Sinha G, Ross T, et al. Severe community-onset pneumonia in healthy adults caused by methicillin-resistant Staphylococcus aureus carrying the Panton-Valentine leukocidin genes. Clin Infect Dis 2005;40:100-107. PMID: 15614698.



21. Gillet Y, Issartel B, Vanhems P, Fournet JC, Lina G, Bes M, et al. Association between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet 2002;359:753-759. PMID: 11888586.


22. Labandeira-Rey M, Couzon F, Boisset S, Brown EL, Bes M, Benito Y, et al. Staphylococcus aureus Panton-Valentine leukocidin causes necrotizing pneumonia. Science 2007;315:1130-1133. PMID: 17234914.


23. Olsen RJ, Kobayashi SD, Ayeras AA, Ashraf M, Graves SF, Ragasa W, et al. Lack of a major role of Staphylococcus aureus Panton-Valentine leukocidin in lower respiratory tract infection in nonhuman primates. Am J Pathol 2010;176:1346-1354. PMID: 20093487.



24. Peyrani P, Allen M, Wiemken TL, Haque NZ, Zervos MJ, Ford KD, et al. Severity of disease and clinical outcomes in patients with hospital-acquired pneumonia due to methicillin-resistant Staphylococcus aureus strains not influenced by the presence of the Panton-Valentine leukocidin gene. Clin Infect Dis 2011;53:766-771. PMID: 21880581.



25. Shindo Y, Ito R, Kobayashi D, Ando M, Ichikawa M, Shiraki A, et al. Risk factors for drug-resistant pathogens in community-acquired and healthcare-associated pneumonia. Am J Respir Crit Care Med 2013;188:985-995. PMID: 23855620.


26. Chalmers JD, Rother C, Salih W, Ewig S. Healthcare-associated pneumonia does not accurately identify potentially resistant pathogens: a systematic review and meta-analysis. Clin Infect Dis 2014;58:330-339. PMID: 24270053.



27. Matsuda S, Ogasawara T, Sugimoto S, Kato S, Umezawa H, Yano T, et al. Prospective open-label randomized comparative, non-inferiority study of two initial antibiotic strategies for patients with nursing- and healthcare-associated pneumonia: guideline-concordant therapy versus empiric therapy. J Infect Chemother 2016;22:400-406. PMID: 27062334.


28. Metersky ML, Frei CR, Mortensen EM. Predictors of Pseudomonas and methicillin-resistant Staphylococcus aureus in hospitalized patients with healthcare-associated pneumonia. Respirology 2016;21:157-163. PMID: 26682638.


29. Jain S, Self WH, Wunderink RG, Fakhran S, Balk R, Bramley AM, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med 2015;373:415-427. PMID: 26172429.



Figure 1
The percentages of identified methicillin-resistant Staphylococcus aureus (MRSA) pathogens in three different risk groups determined using the scoring system.
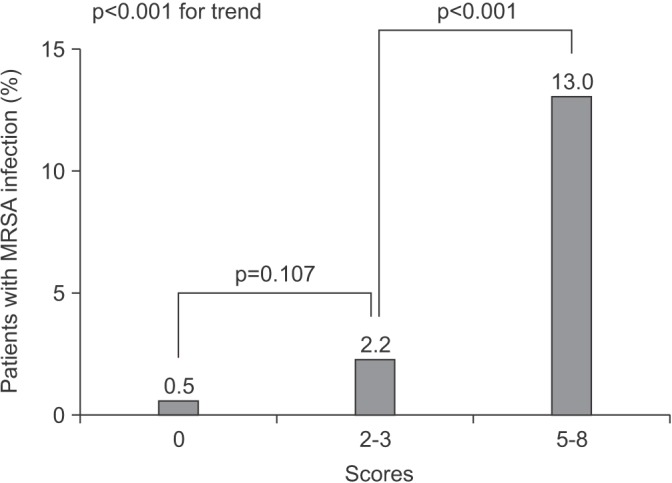
Figure 2
Receiver operating characteristic curves of the scoring system for prediction of methicillin-resistant Staphylococcus aureus infection in elderly patients with community-onset pneumonia.
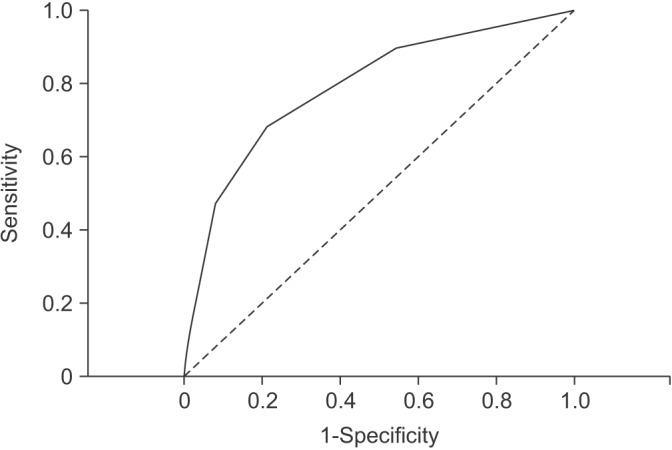
Table 1
Baseline characteristics of elderly patients admitted with community-onset pneumonia
Value are presented as median (interquartile range) or number (%).
*Allowed for overlapping.
MRSA: methicillin-resistant Staphylococcus aureus; CAP: community-acquired pneumonia; HCAP: healthcare-associated pneumonia; CURB-65: Confusion, Urea, Respiratory rate, Blood pressure, Age ≥65; PSI: Pneumonia Severity Index.
Table 2
Microorganisms isolated from elderly patients admitted with community-onset pneumonia, non-MRSA group (n=743)
*Other gram negative species included Escherichia coli, Enterobacter species, Serratia marcescens, and Legionella pneumophilia. †Potentially drug-resistant pathogens included MRSA, Pseudomonas species, Acinetobacter species, Stenotrophomonas maltophilia, and ESBL-producing Enterobacteriaceae.
MRSA: methicillin-resistant Staphylococcus aureus; ESBL: extended-spectrum β-lactamase.


 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Print
Print Download Citation
Download Citation




