 |
 |
| Tuberc Respir Dis > Volume 80(1); 2017 > Article |
|
Abstract
Approximately one in four patients with chronic obstructive pulmonary disease (COPD) have asthmatic features consisting of wheezing, airway hyper-responsiveness or atopy. The Global initiative for Asthma/Globalinitiative for chronic Obstructive Lung Disease committee recently labelled these patients as having asthma-COPD overlap syndrome or ACOS. ACOS also encompasses patients with asthma, ≥40 years of age, who have been cigarette smokers (more than 5-10 pack years) or have had significant biomass exposure, and demonstrate persistent airflow limitation defined as a post-bronchodilator forced expiratory volume in 1 second (FEV1)/forced vital capacity of <70%. Data over the past 30 years indicate that patients with ACOS have greater burden of symptoms including dyspnea and cough and show higher risk of COPD exacerbations and hospitalizations than those with pure COPD or pure asthma. Patients with ACOS also have increased risk of rapid FEV1 decline and COPD mortality. Paradoxically, experimental evidence to support therapeutic decisions in ACOS patients is lacking because traditionally, patients with ACOS have been systematically excluded from therapeutic COPD and asthma trials to maintain homogeneity of the study population. In this study, we summarize the current understanding of ACOS, focusing on definitions, epidemiology and patient prognosis.
Asthma and chronic obstructive pulmonary disease (COPD) are common conditions that impact directly on the airways. The most current estimates indicate that there are 330 million individuals with asthma and 384 million with COPD worldwide1. Korea is no different than most industrialized countries in terms of prevalence of asthma and COPD2,3. According to data from the fourth Korean National Health and Nutrition Survey, which randomly sampled 6,840 Korean adults, 19 years of age and older, in 2008, 13.4% of Korean adults 40 years of age and older had spirometric evidence of airflow limitation, defined by forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) ratio of less than 0.73. Given that there are 24 million Koreans over 40 years of age4, population-based calculations suggest that there are 3.2 million adults living in Korea with fixed airflow limitation, most likely from COPD. The prevalence of asthma is also high in Korean adults 40 years of age and older with approximately 10% of adults 40 to 50 years of age having asthma. Remarkably, among those 70 years of age and older, the prevalence of asthma exceeds >70% in Korea2. With these remarkably high rates of asthma and COPD in the Korean community, just by chance, there will be many patients who have features of both asthma and COPD. With increasing age, the probability of overlap increases as the prevalence of these two conditions rise. Given these high rates of athma and COPD in Korea, the prevalence of asthma-COPD overlap syndrome (ACOS) may be as high as 50% in individuals over the age of 80 with fixed airflow limitation5.
Although asthma and COPD share some similar clinical features including symptoms (e.g., dyspnea, wheezing, and cough) and airflow limitation, pathophysiologically they are distinct disorders. For instance, while airway inflammation is observed in both conditions, in asthma there is a predominance of eosinophils in the airways and in COPD there is a predominance of neutrophils. Furthermore, in asthma there is a lymphocytic bias towards type 2 helper (Th2) cells and Th2 cytokine network; whereas in COPD, there is a bias towards type 1 helper (Th1) and type 1 cytotoxic T-cells6,7. Even with airflow limitation, there are subtle but important differences. In asthma the airflow limitation tends to be episodic with complete or near complete reversible during periods of stability or with treatment6. In contrast, in COPD, the airflow limitation tends to be persistent and often progressive7. Clinical presentation is also typically different. Asthma often develops in childhood, then undergoes remission during adolescence or young adulthood and may return when individuals reach middle age, although in adult onset asthma, symptoms do not typically appear until patients are in their 40s or 50s8. In many cases, asthma coexists with atopy and allergic rhinitis2 though many patients with atopy or allergic rhinitis never develop asthma. COPD, on the other hand, is driven by long-term exposure to cigarette smoke or biomass fuel, though increasingly the role of prior tuberculosis infection is being recognized as an important cofactor for COPD in tuberculosis endemic areas such as Korea and South Africa9,10,11,12. Aside from cigarette smoke and a prior history of tuberculosis, the other major risk factor for COPD is aging. COPD is distinctly unusual before age 30 and its prevalence increases exponentially beyond age 40. Thus, in young adults who present with shortness of breath and airflow limitation, asthma is most likely. However, because long-term asthma or adult-onset asthma can present with fixed airflow limitation, if the same patient presents in his 40s and beyond with these symptoms, diagnostic confusion can arise. Moreover, given the high prevalence of both asthma and COPD in the Korean adult population over 40 years of age, there may be a large number of patients with fixed airflow limitation, who may have both features. Thus, for the “average” clinician, “binning” of patients either to “asthma” or “COPD” categories (but not to both) is often challenging and capricious.
Although some may argue that diagnostic labels are just nomenclature (i.e., just words), in reality, therapeutic implications are vast depending on the diagnosis. Once the patient is labelled with “asthma,” expert guidelines recommend inhaled corticosteroids6 as the first line of therapy with almost no exception. Patients, who are diagnosed with “COPD,” on the other hand, should be given as first line therapy long acting bronchodilators and no inhaled corticosteroids (with almost no exception)7. The dilemma comes when patients have features of both asthma and COPD. What should the practicing clinician do under this scenario? (Figure 1). Regrettably, there is remarkable silence from the experts on the management of patients with both asthma and COPD because there is a marked scarcity of high quality data that have evaluated management strategies on such patients. To date there have been no large Phase III clinical trials that have evaluated novel therapeutics in patients who have both asthma and COPD. Traditionally, to qualify drugs for COPD, drug makers had to systematically exclude patients who have had any significant history of asthma. Some had to even exclude individuals, who did not have a personal history of asthma but demonstrated “large” bronchodilator responses on baseline spirometry13. Similarly, for asthma trials, drug makers had to routinely exclude current smokers and even ex-smokers who had smoked for more than 5 pack-years13. This was done for regulatory reasons and because of the ongoing concern among drug makers that “asthma” drugs would be less effective in populations that are “contaminated” by patients who may also have COPD14.
Recently, there has been a shift in emphasis away from diagnostic disease categories to “treatable traits”15. The problem with this approach is that there is little consensus on what these “treatable traits” are in asthma and COPD and more importantly no consensus on the threshold values that should be used for each of the treatable traits to maximize therapeutic signals (and minimize risks associated with treatment). Take for example peripheral eosinophils in asthma and COPD. Although there is an agreement that individuals who demonstrate “high” peripheral eosinophilia with airways disease should be treated with inhaled corticosteroids, there is no consensus on what threshold value should be used to guide therapy13,16,17. Some have used an absolute peripheral eosinophil count, while others have used percentage of total leukocyte count. Still others have used the upper limit of normal values; whereas others have used lower (arbitrary) thresholds. The lack of consensus (reflecting the paucity of high quality studies that have addressed this issue) creates confusion for the practicing clinicians, preventing them from implementing this approach in clinical practice. There is another major problem of using “treatable trait” approach to airways disease. Patients with COPD, in particular, have multiple comorbidities and non-specific symptoms. The use of the “treatable trait” approach can lead to polypharmacy with drugs of marginal benefit (e.g. statins for treatment of “systemic inflammation”) that may have serious consequences for patients, especially elderly patients18,19. Until these issues are fully resolved, treating the “treatable trait” approach cannot be advocated. Thus, there is a pressing need to define the patients who have both asthma and COPD features and to conduct rigorous clinical trials to ascertain the best management strategies for these patients.
There is no universally accepted definition of ACOS. In response to the diagnostic and therapeutic challenges faced by practicing clinicians who manage patients with airways disease, the Global initiative for Asthma (GINA) and the Global initiative for chronic Obstructive Lung Disease (GOLD) committees jointly created the term asthma-COPD overlap syndrome (ACOS) to acknowledge the daily reality of patients who have features of both asthma and COPD and to begin the conversation that will lead to a “consensus” definition of ACOS20. However, because the GINA/GOLD document on ACOS is a series of checklist of characteristics of ACOS without thresholding, it is not particularly useful as a diagnostic tool for the clinician. Others have offered more practical case definitions of ACOS (Tables 1, 2, 3)16,21. The Spanish Society of Pneumology and Thoracic Surgery (SEPAR) and the Roundtable Groups have endorsed case definitions based on major and minor criteria. The Roundtable group recommends fulfillment of all 3 major criteria for ACOS (1, persistent airflow limitation + 2, ≥10 pack years of smoking or biomass exposure + 3, documented history of asthma or bronchodilator response of 400 mL or greater in those without a prior history of asthma) and at least one minor criterion (1, documented history of atopy or allergic rhinitis; or 2, bronchodilator response of 200 mL or greater on 2 different occasions; or 3, peripheral eosinophil count of 300/µL or greater). SEPAR, on the other hand, recommends the presence of at least one major criterion (history of asthma or bronchodilator response of ≥400 mL) or two minor criteria (1, IgE >100 IU or history of atopy; or 2, bronchodilator response of ≥200 mL on two different occasions or blood eosinophilia of >5%) in the presence of fixed airflow limitation. Using this case definition, Cosio et al.22 examined the prevalence of ACOS in the COPD History Assessment in Spain (CHAIN) cohort and found that 15% of the COPD patients met SEPAR's diagnostic criteria for ACOS. Importantly, they found that patients with ACOS had 2 times the mortality rate at 1 year compared with patients, who did not have ACOS but had COPD only22.
Most of the large data published on ACOS prevalence have used a more convenient definition based on self-report of COPD and asthma or spirometry plus self-report of asthma rather than GINA/GOLD's, SEPAR's or Sin's definitions of ACOS. There have been approximately 17 studies that have examined this relationship23,24,25,26,27,28,29,30,31,32,33,34,35,36 and Alshabanat et al.37 performed a meta-analysis of these 17 studies and found that the pooled prevalence of ACOS was approximately 27% among individuals who had originally been diagnosed with COPD. Although there was some heterogeneity among the included studies, ACOS was generally more prevalent in the younger populations (40 to 60 years of age) compared with the older populations (>60 years of age). There was no consensus on whether there is a sex difference in the rate of ACOS in the community with some studies reporting a female predominance, while others demonstrating no significant differences between men and women37. One major limitation of all of these studies was the reliance on self-report of asthma, which may be susceptible to recall bias.
To address this limitation, Tkacova et al.38 used bronchial hyperresponsiveness to define the “asthmatic” phenotype in patients with COPD. Using the Lung Health Study (LHS) data, which measured bronchial hyperresponsiveness to methacholine in patients with mild to moderate COPD, they found that 24% of these patients demonstrated bronchial hyperresponsiveness as defined by a provocation concentration (PC20) of 4 mg/mL or less to induce at least a 20% fall in FEV1 from baseline values. ACOS defined by bronchial hyperresponsiveness in COPD patients was associated with a faster decline in FEV1 and increased risk of respiratory but not all-cause mortality over 11 years of follow-up. It should be noted, however, that although bronchial hyperresponsiveness is one of the hallmarks (and defining features) of asthma, its pathophysiology may be quite different in COPD than that in asthma. In asthma, for instance, bronchial hyperresponsiveness appears to be driven by underlying eosinophilic airway inflammation and disturbances in airway smooth muscle; whereas in COPD, the main risk factors of bronchial hyperresponsiveness are altered baseline geometry of the airways and smoking39.
To date, the totality of data suggests that one in four patients with COPD have coexisting asthma or “asthmatic” features. What is less known is the prevalence of COPD in those with pre-existing asthma. Population based studies that used spirometry to ascertain individuals with fixed (or persistent) airflow limitation indicates that approximately 25% to 30% of individuals with “COPD” (defined based on post-bronchodilator FEV1/FVC falling below the lower limit of normal values) are lifetime never smokers, representing approximately 7% of the general population40,41. The main risk factors for COPD among never smokers are history of asthma and increasing age. Self-reported history of asthma (which is present in 5% to 10% of the general population aged 40 years and older) increases the risk of COPD by ~4- to 5-fold41. Presence of bronchial hyperresponsiveness, which is as noted earlier one of the hallmark findings in asthma, also increases the risk of incident COPD by 4-fold, independent of other factors including smoking and aging42. In the general population, the population risk factor of asthma (defined either by bronchial hyperresponsiveness or self-report) for incident COPD is approximately 20% to 25% versus smoking, which is associated with a population attributable risk of 38% for incident COPD42.
What is less known is the impact of lifetime cigarette smoking on the incidence of COPD among asthmatics. Smoking is the most important risk factor for accelerated decline for both men and women, such that heavy smokers experience 50 mL/yr decline in FEV1 in men and 32 mL/yr decline in women. Asthma by itself imposes a small excess risk of accelerate decline. Male asthmatics experience on average 40 mL/yr FEV1 decline and female asthmatics experience 28 mL/yr decline. There is an additive effect of smoking and asthma such that male asthmatics who smoke experience a 54 mL/yr decline and female asthmatics who smoke experience a 36 mL/yr decline (normal ~20 to 30 mL/yr decline)43.
Despite this relatively modest excess risk of FEV1 decline imposed by asthma, asthmatics are over-represented in the COPD population by age 60 years because asthmatics in general have smaller lungs at full lung maturity (which occurs between 18 and 25 years of age). Thus, James et al.43 found that at age 60, an “average” white nonsmoking male with asthma in Busselton had FEV1 that was approximately 380 mL lower than a similar non-asthmatic male (we will call the latter individual the “reference” male). Interestingly, a similar (heavy) smoking (non-asthmatic) male had FEV1 that was 190 mL lower than the reference male. If the heavy smoker was also an asthmatic, his FEV1 was 560 mL lower than of the reference male.
The additive effects of smoking on asthmatics were well described in the Busselton Study, which was discussed earlier and in the Copenhagen City Heart Study (CCHS). CCHS was a prospective population-based study, which began in 1976 and in which 50% of the study subjects were smokers at enrollment and ~3% were asthmatics. The average FEV1 decline in those with asthma was 38 mL/yr versus 22 mL/yr without asthma. As with the Busselton Study, smoking had an additive effect in accelerating the decline in FEV1 over 15 years by increasing the rate of FEV1 decline by 10 to 20 mL/yr44. Together, these data suggest that asthmatics have increased risk of “COPD” (defined by post-bronchodilator FEV1/FVC below 70% or the lower limit of normal on spirometry) between 40 and 80 years of age through two independent mechanisms: (1) reduced lung growth in childhood and (2) accelerated decline in FEV1 during adulthood. Smoking amplifies this risk (in an additive fashion) by accelerating FEV1 decline. While there is tremendous variation, on average, asthmatics experience 10 mL/yr excess decline in FEV1 versus non-asthmatics and smoking adds another 10 to 20 mL/year decline in FEV1. Thus, by age 60, one in three to one in two asthmatics who smoke through their lifetime will develop COPD (vs. 15% to 20% of non-asthmatics who smoke).
In general, patients with ACOS appear to be more symptomatic and have worse health outcomes compared with patients with asthma or COPD alone, though there is considerable heterogeneity of data across studies. In the EPI-SCAN Study, for instance, which was a population-based Spanish cohort that examined 3,885 individuals 40 to 80 years of age, Miravitlles et al.32 showed that compared with individuals with COPD alone, those who had both COPD (based on spirometry) and asthma (based on self-report) had more dyspnea, lower 6-minute walk distance, and worse health status (as measured on St. George's Respiratory Questionnaire). In the PLATINO Study, which was a population-based study in five Latin American countries, individuals with ACOS had more respiratory symptoms, worse lung function and were 2.1 times more likely to experience an exacerbation and 4 times more likely to become hospitalized than those with COPD alone24. Similarly, the COPDgene Study showed that individuals with COPD (defined by spirometry) and asthma (defined by selfreport of physician diagnosis of asthma) were diagnosed with COPD at an earlier age, with lower lifetime smoking intensity and reported more symptoms and worse quality of life compared with individuals with COPD without asthma. Importantly, individuals with ACOS were 3.6 times more likely to be a “frequent” exacerbator (defined as having 2 or more exacerbations per year) and to experience severe exacerbations requiring hospitalization or ventilatory support in the intensive care units27. These findings have been largely recapitulated in other parts of the world including Asia. Rhee et al.36 in Korea have shown that individuals with ACOS were likely to use emergency rooms and be hospitalized for their respiratory condition compared with individuals with COPD alone. The two large systematic reviews and meta-analyses conducted on ACOS have affirmed these findings by demonstrating that patients with ACOS have greater symptomatic burden and require greater health care utilization including emergency department visits and hospitalization than individuals with COPD alone37,45.
One interesting phenotype associated with ACOS is atopy and allergic rhinitis. De Marco et al.46 found that individuals who had ACOS (defined by self-report of asthma and COPD) were two times more likely to report allergic rhinitis compared with individuals who had COPD only. However, because most patients with allergic rhinitis or atopy do not develop airflow limitation, the presence of these conditions is not very useful in defining ACOS in clinical practice.
Although the exact pathogenesis of ACOS is unknown, there is general belief that ACOS is associated with more “airway” disease than pure COPD. Pathologically, COPD is typically characterized by airway inflammation and remodeling (thickening of wall) involving small airways (which are less than 2 mm in diameter) and destruction of respiratory bronchioles and distal parenchyma, leading to emphysematous changes in the lungs47. Both the changes in the small airways and emphysema contribute to airflow limitation. As noted previously, cigarette smoke induces centrilobular emphysema as well as airway remodeling; whereas biomass smoke appears to predominantly contribute to airway remodeling (and less to emphysema)48. Alpha-1-antitrypsin deficiency, which is the only known genetic cause of COPD, leads to panlobular emphysema and airway remodeling (though the airway remodeling is less prominent than that caused by cigarette smoke)49. Asthma, on the other hand, is largely characterized by airway inflammation and remodeling involving both the larger and smaller airways. Although parenchymal changes can be found in asthmatics, they are rare and are usually associated with severe (life-threatening) disease or acute exacerbations50. Superficially, the inflammatory drivers of airway inflammation appear to be quite different than those of COPD. It is widely accepted that the inflammatory process of asthma is related to eosinophilic and Th2 pathways; whereas in COPD, neutrophils are thought to play the predominant role. However, with increasing scrutiny, there are emerging data to indicate that this dogma oversimplifies reality. Many asthmatics, especially older ones with moderate to severe symptoms, have an abundance of neutrophils in their airways and as many as 30% to 40% of COPD patients demonstrate elevated eosinophil count in both sputum and in peripheral blood51. Moreover, the uniqueness of Th2 bias in asthma is now questioned as COPD airways may also demonstrate the “asthma” biosignature. Christenson et al.52, for example, used transcriptomics data from bronchial brushes of medium sized and small airways of COPD patients and found that the “asthma” genomic signature consisting mostly of genes involved in the Th2 inflammatory pathway was enriched in COPD airways compared with healthy controls and smokers without COPD. Most importantly, the COPD patients who had Th2-high gene expression profile on bronchial brushes demonstrated greater bronchodilator reversibility, peripheral and tissue eosinophilia, and a more favorable response to inhaled corticosteroids than COPD patients who did not have the Th2 biosignature52. It is tempting to speculate that these COPD patients who have enriched Th2 biosignature in their airways may represent those with ACOS. Additional studies will be needed to validate this hypothesis.
ACOS patients may also represent those with more airway disease than emphysema even among smokers. The COPDgene investigators carefully phenotyped patients with ACOS versus those with pure COPD and found that ACOS patients had more severe and more frequent respiratory exacerbations, less emphysema and greater airway wall thickness on computed tomography (CT) compared to subjects with COPD alone53. Similarly, Gao et al.54 using inspiratory and expiratory CT images, showed that patients with ACOS demonstrated less emphysema burden and less gas trapping following bronchodilators than those with pure COPD. Interestingly, Hardin et al.53 performed genetic analysis of ACOS and found significant hits in genes for CSMD1, SOX5, and GPR65. Together, these data suggest that ACOS is associated with molecular drivers of asthma and that cigarette smoke amplifies these drivers.
Sorino et al.55 followed patients with ACOS, COPD, and asthma patients for 15 years and found that compared with individuals in the community without any of these airway disorders, those with ACOS had 1.83-fold increase in mortality (p<0.0001), those with “pure” COPD had 2.31-fold increase (p<0.0001) and those with “pure” asthma had 1.19-fold increase in risk of total mortality (p=0.085). In the CCHS, ACOS was subdivided into two groups: ACOS with early onset-asthma (asthma onset before 40 years) and ACOS with late onset-asthma (asthma onset ≥40 years of age). They found that 36% of study participants with any airway disease had ACOS. Compared with never smokers without asthma or COPD, total mortality was increased by 1.81 fold in late onset asthma ACOS (p<0.0001), by 1.44 in early onset asthma ACOS (p=0.03), by 1.73 in “pure” COPD (p<0.0001), and by 1.05 in “pure” asthma (p=0.73). For respiratory mortality, the corresponding hazard ratios (HRs) are 6.36 in late onset asthma ACOS (p<0.0001); 2.30 in early onset ACOS (p=0.06), 3.69 in pure COPD (p<0.0001), and 2.58 in pure asthma (p=0.01)56. Compared with “pure” COPD, late onset asthma ACOS was associated with greater risk of total mortality (HR, 1.39; p=0.001) and respiratory mortality (HR, 3.51; p<.0001). In contrast, there was no significant mortality difference between pure COPD and early onset asthma ACOS. Individuals with late onset asthma ACOS had the highest rate of decline in FEV1 at 49.6 mL/yr, followed by “pure” COPD at 39.5 mL/yr, early onset asthma ACOS at 27.3 mL/yr and healthy ever or never smokers at 21 mL/yr56. Together, these data suggest that ACOS, especially when asthma is diagnosed later on in life, is associated with worse prognosis than even with pure COPD, driven largely by increased risk of progression of their lung disease and respiratory mortality.
As noted previously, there have been no large scale therapeutic trials on patients with ACOS. It is generally believed, though not proven, that patients with ACOS would respond to better to inhaled corticosteroids than those with pure COPD. Moreover, as noted earlier, ACOS patients in general have more symptoms (of dyspnea and cough), worse health status and are at increased risk of exacerbations compared with patients with pure asthma or pure COPD. Thus, they may require more intensive management. Some experts have suggested inhaled corticosteroids/long acting beta2 agonist combination (ICS/LABA) as the first line therapy for ACOS, followed by the addition of a long acting anticholinergic if the patient remains persistently symptomatic with ICS/LABA combination57. This notion is supported by the GLUCOLD Study58, which evaluated the effects of ICS and ICS/LABA combination therapy on FEV1 decline and bronchial hyperresponsiveness. They found in this relatively small study was that 30 months of ICS or ICS/LABA therapy significantly reduced the rate of decline in FEV158. What was different about this cohort versus other much larger cohorts such as the Lung Health Study 2 (LHS2) which also evaluated the effects of ICS on FEV1 decline (and showed no impact), was that the geometric mean of the provocation concentration (PC20) in GLUCOLD was 0.7 mg/mL versus ~8 to 10 mg/mL in the LHS2 study59. These data suggest that COPD patients who also have bronchial hyperresponsiveness (BHR) are more likely to “respond” to ICS or ICS/LABA compared with COPD patients who do not demonstrate BHR. Another approach is to use peripheral eosinophil count to guide the choice of treatment. However, as noted earlier, while the use of peripheral eosinophil count is appealing and intuitive, currently there is no consensus on the thresholds that should be used to target patients with ICS or ICS/LABA. Nevertheless, there are some promising retrospective data showing that COPD patients with “elevated” peripheral eosinophil counts respond better to ICS or ICS/LABA combination than those with non-elevated peripheral eosinophil counts16,17,60. Peripheral eosinophil count may also associate with risk of pneumonia related to ICS use61, which if confirmed in a prospective study, may provide clarity on the risk-benefits of ICS therapy in ACOS patients.
While the role of ICS is debatable, there is general consensus that symptomatic ACOS patients should receive a long-acting bronchodilator. Which long-acting bronchodilators are optimal in ACOS is matter of debate. In general, long acting muscarinic antagonists coax a greater improvement in FEV1 and larger reduction in the risk of exacerbations than LABAs in COPD62. However, comparative studies have not been performed in patients with ACOS. Thus, firm recommendations are not possible.
Until high quality data become available in ACOS, it may be prudent to use low-dose inhaled corticosteroids and a long-acting bronchodilator as the first line treatment for patients with ACOS. There is no reason to believe that patients with ACOS would have differential risk of pneumonia related to ICS compared with those with pure COPD. Thus, ICS should be used with caution in ACOS patients whose FEV1 is less than 50% of predicted63. Similar to patients with pure COPD or elderly patients with asthma, patients with ACOS should be vaccinated annually for influenza and every 5 to 10 years with pneumococcus and provided with the resources to foster smoking cessation. Figure 1 depicts a practical way of evaluating and managing patients with ACOS.
One in four patients with COPD have ACOS. Patients with ACOS have a great burden of symptoms and poor health status. They are at increased risk of exacerbations and hospitalizations and experience accelerated decline in lung function compared with patients with pure COPD or asthma. Moreover, they are at the highest risk of COPD mortality. Despite this large burden of disease and poor prognosis of these patients, there is little high quality evidence in the literature to guide therapeutic decisions in ACOS patients. While there is general consensus that inhaled corticosteroids may be effective in these patients, there is surprisingly a paucity of data to support this belief. However, until more (solid) data are available, inhaled corticosteroids, along with long acting bronchodilators are reasonable first line therapies for patients with ACOS.
Given the large burden of disease in ACOS patients, there is a pressing need to conduct high quality therapeutic trials to determine the most optimal management strategies for these patients. There is also an urgent need of easy, inexpensive biomarkers to accurately identify ACOS patients and to guide therapeutic choices. The consensus definitions by SEPAR and the Roundtable groups will enable the conduct of these crucial studies.
Acknowledgments
D. Sin is a Tier 1 Canada Research Chair and is supported by funding from the Canadian Institutes of Health Research and Genome Canada.
References
1. Adeloye D, Chua S, Lee C, Basquill C, Papana A, Theodoratou E, et al. Global and regional estimates of COPD prevalence: systematic review and meta-analysis. J Glob Health 2015;5:020415PMID: 26755942.




2. Park HS, Choi GS, Cho JS, Kim YY. Epidemiology and current status of allergic rhinitis, asthma, and associated allergic diseases in Korea: ARIA Asia-Pacific workshop report. Asian Pac J Allergy Immunol 2009;27:167-171. PMID: 19839504.

3. Yoo KH, Kim YS, Sheen SS, Park JH, Hwang YI, Kim SH, et al. Prevalence of chronic obstructive pulmonary disease in Korea: the fourth Korean National Health and Nutrition Examination Survey, 2008. Respirology 2011;16:659-665. PMID: 21342331.


4. Organization for Economic Co-operation and Development. OECD.stat. Total population by sex and age [Internet]. Paris: Organization for Economic Co-operation and Development; 2016. cited 2016 Aug 2. Available from: http://stats.oecd.org/index.aspx?DataSetCode=RPOP#.
5. Soriano JB, Davis KJ, Coleman B, Visick G, Mannino D, Pride NB. The proportional Venn diagram of obstructive lung disease: two approximations from the United States and the United Kingdom. Chest 2003;124:474-481. PMID: 12907531.


6. Reddel HK, Bateman ED, Becker A, Boulet LP, Cruz AA, Drazen JM, et al. A summary of the new GINA strategy: a roadmap to asthma control. Eur Respir J 2015;46:622-639. PMID: 26206872.



7. Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013;187:347-365. PMID: 22878278.


8. Gibson PG, McDonald VM, Marks GB. Asthma in older adults. Lancet 2010;376:803-813. PMID: 20816547.


9. Eisner MD, Anthonisen N, Coultas D, Kuenzli N, Perez-Padilla R, Postma D, et al. An official American Thoracic Society public policy statement: novel risk factors and the global burden of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2010;182:693-718. PMID: 20802169.


10. Barnes PJ, Burney PG, Silverman EK, Celli BR, Vestbo J, Wedzicha JA, et al. Chronic obstructive pulmonary disease. Nat Rev Dis Primers 2015;1:15076PMID: 27189863.


11. van Gemert F, Kirenga B, Chavannes N, Kamya M, Luzige S, Musinguzi P, et al. Prevalence of chronic obstructive pulmonary disease and associated risk factors in Uganda (FRESH AIR Uganda): a prospective cross-sectional observational study. Lancet Glob Health 2015;3:e44-e51. PMID: 25539969.


12. Po JY, FitzGerald JM, Carlsten C. Respiratory disease associated with solid biomass fuel exposure in rural women and children: systematic review and meta-analysis. Thorax 2011;66:232-239. PMID: 21248322.


13. Sin DD, Miravitlles M, Mannino DM, Soriano JB, Price D, Celli BR, et al. What is asthma-COPD overlap syndrome? Towards a consensus definition from a round table discussion. Eur Respir J 2016;48:664-673. PMID: 27338195.


14. Chalmers GW, Macleod KJ, Little SA, Thomson LJ, McSharry CP, Thomson NC. Influence of cigarette smoking on inhaled corticosteroid treatment in mild asthma. Thorax 2002;57:226-230. PMID: 11867826.



15. Agusti A, Bel E, Thomas M, Vogelmeier C, Brusselle G, Holgate S, et al. Treatable traits: toward precision medicine of chronic airway diseases. Eur Respir J 2016;47:410-419. PMID: 26828055.


16. Siddiqui SH, Guasconi A, Vestbo J, Jones P, Agusti A, Paggiaro P, et al. Blood eosinophils: a biomarker of response to extrafine beclomethasone/formoterol in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2015;192:523-525. PMID: 26051430.



17. Pavord ID, Lettis S, Locantore N, Pascoe S, Jones PW, Wedzicha JA, et al. Blood eosinophils and inhaled corticosteroid/long-acting beta-2 agonist efficacy in COPD. Thorax 2016;71:118-125. PMID: 26585525.


18. Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA 2015;314:1818-1831. PMID: 26529160.



19. Scott IA, Hilmer SN, Reeve E, Potter K, Le Couteur D, Rigby D, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med 2015;175:827-834. PMID: 25798731.


20. GINA/GOLD Joint Report. 2015 Asthma, COPD and Asthma-COPD overlap syndrome (ACOS) [Internet]. Bethesda: Global Initiative for Asthma; 2016. cited 2016 Aug 1. Available from: http://ginasthma.org/asthma-copd-and-asthma-copdoverlap-syndrome-acos/.
21. Soler-Cataluna JJ, Cosio B, Izquierdo JL, Lopez-Campos JL, Marin JM, Aguero R, et al. Consensus document on the overlap phenotype COPD-asthma in COPD. Arch Bronconeumol 2012;48:331-337. PMID: 22341911.


22. Cosio BG, Soriano JB, Lopez-Campos JL, Calle-Rubio M, Soler-Cataluna JJ, de-Torres JP, et al. Defining the asthma-COPD overlap syndrome in a COPD cohort. Chest 2016;149:45-52. PMID: 26291753.


23. Shirtcliffe P, Marsh S, Travers J, Weatherall M, Beasley R. Childhood asthma and GOLD-defined chronic obstructive pulmonary disease. Intern Med J 2012;42:83-88. PMID: 20403069.


24. Menezes AM, Montes de Oca M, Perez-Padilla R, Nadeau G, Wehrmeister FC, Lopez-Varela MV, et al. Increased risk of exacerbation and hospitalization in subjects with an overlap phenotype: COPD-asthma. Chest 2014;145:297-304. PMID: 24114498.


25. Marsh SE, Travers J, Weatherall M, Williams MV, Aldington S, Shirtcliffe PM, et al. Proportional classifications of COPD phenotypes. Thorax 2008;63:761-767. PMID: 18728201.



26. Kauppi P, Kupiainen H, Lindqvist A, Tammilehto L, Kilpelainen M, Kinnula VL, et al. Overlap syndrome of asthma and COPD predicts low quality of life. J Asthma 2011;48:279-285. PMID: 21323613.


27. Hardin M, Silverman EK, Barr RG, Hansel NN, Schroeder JD, Make BJ, et al. The clinical features of the overlap between COPD and asthma. Respir Res 2011;12:127PMID: 21951550.




28. Izquierdo-Alonso JL, Rodriguez-Gonzalezmoro JM, de Lucas-Ramos P, Unzueta I, Ribera X, Anton E, et al. Prevalence and characteristics of three clinical phenotypes of chronic obstructive pulmonary disease (COPD). Respir Med 2013;107:724-731. PMID: 23419828.


29. Johannessen A, Omenaas E, Bakke P, Gulsvik A. Incidence of GOLD-defined chronic obstructive pulmonary disease in a general adult population. Int J Tuberc Lung Dis 2005;9:926-932. PMID: 16104642.

30. Danielsson P, Olafsdottir IS, Benediktsdottir B, Gislason T, Janson C. The prevalence of chronic obstructive pulmonary disease in Uppsala, Sweden: the Burden of Obstructive Lung Disease (BOLD) study: cross-sectional population-based study. Clin Respir J 2012;6:120-127. PMID: 21651748.


31. Methvin JN, Mannino DM, Casey BR. COPD prevalence in southeastern Kentucky: the burden of lung disease study. Chest 2009;135:102-107. PMID: 18689574.


32. Miravitlles M, Soriano JB, Ancochea J, Munoz L, Duran-Tauleria E, Sanchez G, et al. Characterisation of the overlap COPD-asthma phenotype: focus on physical activity and health status. Respir Med 2013;107:1053-1060. PMID: 23597591.


33. Zhou Y, Wang C, Yao W, Chen P, Kang J, Huang S, et al. COPD in Chinese nonsmokers. Eur Respir J 2009;33:509-518. PMID: 19251797.


34. Jyrki-Tapani K, Sovijarvi A, Lundback B. Chronic obstructive pulmonary disease in Finland: prevalence and risk factors. COPD 2005;2:331-339. PMID: 17146998.


35. Fabbri LM, Romagnoli M, Corbetta L, Casoni G, Busljetic K, Turato G, et al. Differences in airway inflammation in patients with fixed airflow obstruction due to asthma or chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2003;167:418-424. PMID: 12426229.


36. Rhee CK, Yoon HK, Yoo KH, Kim YS, Lee SW, Park YB, et al. Medical utilization and cost in patients with overlap syndrome of chronic obstructive pulmonary disease and asthma. COPD 2014;11:163-170. PMID: 24111662.


37. Alshabanat A, Zafari Z, Albanyan O, Dairi M, FitzGerald JM. Asthma and COPD overlap syndrome (ACOS): a systematic review and meta analysis. PLoS One 2015;10:e0136065PMID: 26336076.



38. Tkacova R, Dai DL, Vonk JM, Leung JM, Hiemstra PS, van den Berge M, et al. Airway hyperresponsiveness in chronic obstructive pulmonary disease: a marker of asthma-chronic obstructive pulmonary disease overlap syndrome? J Allergy Clin Immunol 2016;138:1571-1579. PMID: 27345171.


39. Jones RL, Noble PB, Elliot JG, James AL. Airway remodelling in COPD: it's not asthma! Respirology 2016;21:1347-1356. PMID: 27381663.


40. Tan WC, Sin DD, Bourbeau J, Hernandez P, Chapman KR, Cowie R, et al. Characteristics of COPD in never-smokers and ever-smokers in the general population: results from the Can-COLD study. Thorax 2015;70:822-829. PMID: 26048404.


41. Thomsen M, Nordestgaard BG, Vestbo J, Lange P. Characteristics and outcomes of chronic obstructive pulmonary disease in never smokers in Denmark: a prospective population study. Lancet Respir Med 2013;1:543-550. PMID: 24461615.


42. de Marco R, Accordini S, Marcon A, Cerveri I, Anto JM, Gislason T, et al. Risk factors for chronic obstructive pulmonary disease in a European cohort of young adults. Am J Respir Crit Care Med 2011;183:891-897. PMID: 20935112.


43. James AL, Palmer LJ, Kicic E, Maxwell PS, Lagan SE, Ryan GF, et al. Decline in lung function in the Busselton Health Study: the effects of asthma and cigarette smoking. Am J Respir Crit Care Med 2005;171:109-114. PMID: 15486340.


44. Lange P, Parner J, Vestbo J, Schnohr P, Jensen G. A 15-year follow-up study of ventilatory function in adults with asthma. N Engl J Med 1998;339:1194-1200. PMID: 9780339.


45. Nielsen M, Barnes CB, Ulrik CS. Clinical characteristics of the asthma-COPD overlap syndrome: a systematic review. Int J Chron Obstruct Pulmon Dis 2015;10:1443-1454. PMID: 26251584.


46. de Marco R, Pesce G, Marcon A, Accordini S, Antonicelli L, Bugiani M, et al. The coexistence of asthma and chronic obstructive pulmonary disease (COPD): prevalence and risk factors in young, middle-aged and elderly people from the general population. PLoS One 2013;8:e62985PMID: 23675448.



47. Hogg JC. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet 2004;364:709-721. PMID: 15325838.


48. Camp PG, Ramirez-Venegas A, Sansores RH, Alva LF, Mc-Dougall JE, Sin DD, et al. COPD phenotypes in biomass smoke-versus tobacco smoke-exposed Mexican women. Eur Respir J 2014;43:725-734. PMID: 24114962.


49. McDonough JE, Yuan R, Suzuki M, Seyednejad N, Elliott WM, Sanchez PG, et al. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N Engl J Med 2011;365:1567-1575. PMID: 22029978.



50. Gelb AF, Yamamoto A, Verbeken EK, Nadel JA. Unraveling the pathophysiology of the asthma-COPD overlap syndrome: unsuspected mild centrilobular emphysema Is responsible for loss of lung elastic recoil in never smokers with asthma with persistent expiratory airflow limitation. Chest 2015;148:313-320. PMID: 25950858.


51. Singh D, Kolsum U, Brightling CE, Locantore N, Agusti A, Tal-Singer R, et al. Eosinophilic inflammation in COPD: prevalence and clinical characteristics. Eur Respir J 2014;44:1697-1700. PMID: 25323230.


52. Christenson SA, Steiling K, van den Berge M, Hijazi K, Hiemstra PS, Postma DS, et al. Asthma-COPD overlap: clinical relevance of genomic signatures of type 2 inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2015;191:758-766. PMID: 25611785.



53. Hardin M, Cho M, McDonald ML, Beaty T, Ramsdell J, Bhatt S, et al. The clinical and genetic features of COPD-asthma overlap syndrome. Eur Respir J 2014;44:341-350. PMID: 24876173.



54. Gao Y, Zhai X, Li K, Zhang H, Wang Y, Lu Y, et al. Asthma COPD overlap syndrome on CT densitometry: a distinct phenotype from COPD. COPD 2016;13:471-476. PMID: 26742511.


55. Sorino C, Pedone C, Scichilone N. Fifteen-year mortality of patients with asthma-COPD overlap syndrome. Eur J Intern Med 2016;34:72-77. PMID: 27357368.


56. Lange P, Colak Y, Ingebrigtsen TS, Vestbo J, Marott JL. Long-term prognosis of asthma, chronic obstructive pulmonary disease, and asthma-chronic obstructive pulmonary disease overlap in the Copenhagen City Heart study: a prospective population-based analysis. Lancet Respir Med 2016;4:454-462. PMID: 27061878.


57. Castiglia D, Battaglia S, Benfante A, Sorino C, Scichilone N. Pharmacological management of elderly patients with asthma-chronic obstructive pulmonary disease overlap syndrome: room for speculation? Drugs Aging 2016;33:375-385. PMID: 27138954.



58. Lapperre TS, Snoeck-Stroband JB, Gosman MM, Jansen DF, van Schadewijk A, Thiadens HA, et al. Effect of fluticasone with and without salmeterol on pulmonary outcomes in chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med 2009;151:517-527. PMID: 19841453.


59. Lung Health Study Research Group. Effect of inhaled triamcinolone on the decline in pulmonary function in chronic obstructive pulmonary disease. N Engl J Med 2000;343:1902-1909. PMID: 11136260.


60. Park HY, Lee H, Koh WJ, Kim S, Jeong I, Koo HK, et al. Association of blood eosinophils and plasma periostin with FEV1 response after 3-month inhaled corticosteroid and long-acting beta2-agonist treatment in stable COPD patients. Int J Chron Obstruct Pulmon Dis 2016;11:23-30. PMID: 26730185.


61. Pavord ID, Lettis S, Anzueto A, Barnes N. Blood eosinophil count and pneumonia risk in patients with chronic obstructive pulmonary disease: a patient-level meta-analysis. Lancet Respir Med 2016;4:731-741. PMID: 27460163.


62. Vogelmeier C, Hederer B, Glaab T, Schmidt H, Rutten-van Molken MP, Beeh KM, et al. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med 2011;364:1093-1103. PMID: 21428765.


63. Sin DD, Tashkin D, Zhang X, Radner F, Sjobring U, Thoren A, et al. Budesonide and the risk of pneumonia: a meta-analysis of individual patient data. Lancet 2009;374:712-719. PMID: 19716963.


Figure 1
Some helpful tips for the busy clinicians. ACOS: asthma-COPD overlap syndrome; COPD: chronic obstructive pulmonary disease; BDR: bronchodilator response; SEPAR: Spanish Society of Pneumology and Thoracic Surgery.
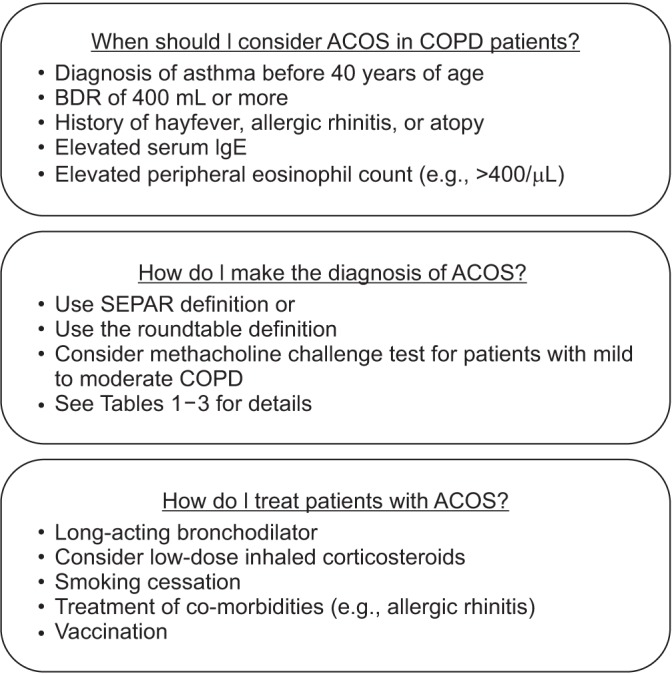
Table 1
Proposed definitions of ACOS: modified GINA/GOLD definition of ACOS
Table 2
Modified SEPAR definition of ACOS21
Of patients with persistent airflow limitation, FEV1/FVC below lower limit of normal or 0.7, ACOS is considered if at least one major criterion or two minor criteria are met.
SEPAR: Spanish Society of Pneumology and Thoracic Surgery; ACOS: asthma-COPD overlap syndrome; COPD: chronic obstructive pulmonary disease; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity.
Table 3
Definition of ACOS from ATS Roundtable Discussions13
To fulfill ACOS, the patient must have all three major criteria and at least one minor criterion.
ACOS: asthma-COPD overlap syndrome; COPD: chronic obstructive pulmonary disease; ATS: American Thoracic Society; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; LLN: lower limit of normal; BDR: bronchodilator response.
- TOOLS


 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Print
Print Download Citation
Download Citation




