Pulmonary Pneumatocele in a Pneumonia Patient Infected with Extended-Spectrum β-Lactamase Producing Proteus mirabilis
Article information
Abstract
Pulmonary pneumatoceles are air-filled thin-walled spaces within the lung and are rare in adult cases of pneumonia. We report the case of a 74-year-old male who was admitted with a cough and sputum production. He had been treated with oral dexamethasone since a brain tumorectomy 6 months prior. Contrast-enhanced computed tomography (CT) of the chest revealed a large pneumatocele in the right middle lobe and peripheral pneumonic consolidation. Bronchoalveolar lavage was performed; cultures identified extended-spectrum β-lactamase (ESBL) producing Proteus mirabilis. A 4-week course of intravenous ertapenem was administered, and the pneumatocele with pneumonia resolved on follow-up chest CT. To the best of our knowledge, this is the first reported case of pulmonary pneumatocele caused by ESBL-producing P. mirabilis associated with pneumonia.
Introduction
Pulmonary pneumatoceles are thin-walled gas-filled cavitary lesions of the lung parenchyma1, and are common in infants and young children with pneumonia, but unusual in adults2. Most often, pneumatoceles develop as complications of acute pneumonia, commonly caused by Staphylococcus aureus34. Pneumatoceles are also caused by other organisms such as gram-negative bacilli (especially pseudomonas ), but reports of pneumatoceles caused by Proteus mirabilis are few15.
P. mirabilis is often associated with contamination and colonization, but it only occasionally associated with severe infections6. Antimicrobial resistance has been reported increasingly for P. mirabilis, and increased resistance of this species to β-lactams, aminoglycosides, and quinolones has become of great concern67.
We here describe a case of pulmonary pneumatocele caused by P. mirabilis in a patient with pneumonia; the organism produced an extended-spectrum β-lactamase (ESBL).
Case Report
A 74-year-old male was admitted with a 2-week history of cough and sputum production. He exhibited low-grade fever and general weakness. His medical history included a brain tumor; tumorectomy of the right frontal cortex and basal ganglia had been performed, followed by concurrent chemoradiation six months prior. After the tumorectomy, he received oral dexamethasone (3 mg/day) prescribed by another hospital. Recently, he had become bedridden and state reported irregular aspiration signs.
On physical examination, he was alert. His breathing sounds were coarse, with a crackle on the right lower anterior aspect of the chest. The abdomen and other organs were normal. All vital signs were stable, except for a low-grade fever. Laboratory data were as follows: arterial blood gas analysis pH, 7.51; PaCO2, 30 mm Hg; PaO2, 75 mm Hg; HCO3-, 23.9 mmol/L; SaO2, 96%; leukocyte count, 10,740/mm3 (segmented neutrophils 95.1%); hemoglobin, 9.2 g/dL; hematocrit, 28.0%; mean corpuscular volume, 100.4 fl; mean corpuscular hemoglobin concentration, 32.9 g/dL; platelet count, 124,000/mm3; C-reactive protein, 7.84 mg/dL; total protein, 5.0 g/dL; and albumin, 2.3 g/dL. Chest radiograph using an anterior-posterior view revealed a cystic lesion in the right medial aspect of the lower lung zone (Figure 1A). Contrast-enhanced computed tomography (CT) of the chest revealed a 5.6×2.5-cm-sized, well-defined, cystic lesion with peripheral enhancement in the right middle lobe, abutting to right minor fissure (Figure 1B-D).
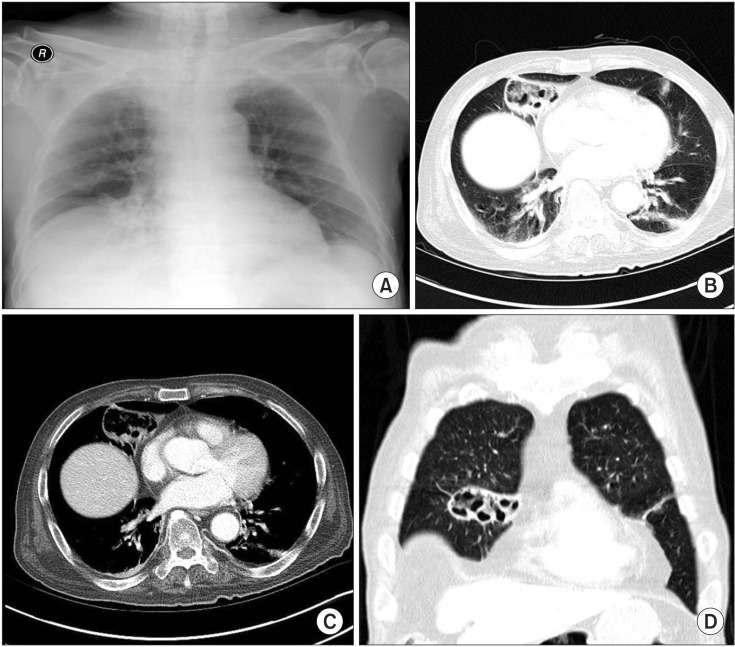
Initial chest radiograph and chest computed tomography. (A) Chest radiograph showing cystic lesion in the medial aspect of right lower lung zone. (B-D) Contrast-enhanced computed tomography showing an approximately 5.6×2.5-cm region of peripheral consolidation, with a central emphysematous change lesion in the right middle lobe, abutting to right minor fissure.
Empirical intravenous antibiotic therapy (piperacillin-tazobactam and levofloxacin) was initiated immediately. An initial sputum culture was negative, as was a sputum acid-fast bacilli (AFB) smear. On the third hospital day, the patient underwent flexible bronchoscopy for microbial evaluation. No endobronchial lesion was apparent, although purulent secretions were noted in the right middle and right lower lobar bronchus. Bronchoalveolar lavage (BAL) was performed in the medial segmental bronchus of the right middle lobe. Analysis of BAL revealed the following: white blood cell count, 4,300/mm3 (segmented neutrophils 94%, lymphocytes 3%, other cells 2%); gram-negative rods, apparent; aspergillus Ag/Ab (IgG), negative/negative; and AFB smear and culture test, negative. On the sixth hospital day, P. mirabilis was identified as the causative organism by culture from BAL fluid. The microbe was subjected to antibiotic sensitivity testing, including ESBL-producing detection. The bacterium was resistant to methicillin and ciprofloxacin and was ESBL-positive, but was sensitive to ertapenem; the antibiotic regimen was therefore changed to intravenous ertapenem. After 4 weeks ertapenem administration, improvement was detected on clinical finding, laboratory test, and radiologic image, the patient was discharged. At the 2-month follow-up, chest radiography and chest CT indicated resolution of the pneumatocele and pneumonia in the right middle lobe and reduced atelectasis (with consolidation) in the right lower lobe (Figure 2A-C).
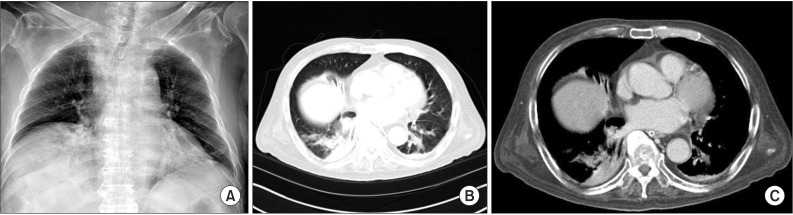
Chest radiograph and chest computed tomography findings after treatment. (A) Chest radiograph taken 2 months after commencement of antibiotic treatment showing resolution of the cystic lesion in the right lower lung zone. (B, C) Contrast-enhanced computed tomography taken 2 months after commencement of antibiotic treatment showing improvement of the lesion in the right middle lobe.
Discussion
The genus Proteus currently consists of five named species (P. mirabilis, P. penneri, P. vulgaris, P. myxofaciens, and P. hauseri) and three unnamed genomospecies (Proteus genomospecies 4, 5, and 6)8. P. mirabilis is one of the most common gram-negative pathogens in clinical specimens and can cause a variety of community or hospital-acquired illnesses6. P. mirabilis is not very virulent and is typically considered only to contaminate and colonize wounds, but not to cause serious infection. However, systemic P. mirabilis infections often develop opportunistically in patients with markedly reduced immune capacities, causing urinary tract, biliary tract, and wound infections and peritonitis7. Classically P. mirabilis is intrinsically resistant to nitrofurantoin and tetracycline, but susceptible to beta-lactams, aminoglycosides, fluoroquinolones, and trimethoprim-sulfamethoxazole78.
ESBL expression was initially most prevalent in Klebsiella pneumoniae and Escherichia coli, but is now emerging in other enterobacterial species, including P. mirabilis9. Over the last few years, ESBL-positive P. mirabilis isolates have been identified worldwide, composing up to 50% of all isolates in certain areas6.
P. mirabilis very rarely causes respiratory infections including pneumonia71011. P. mirabilis pneumonia is sporadic, and usually not associated with hospital outbreaks. However, Okimoto et al.11 reported that 13 cases of levofloxacin-resistant P. mirabilis pneumonia occurred in one hospital. Kim et al.12 reported a nosocomial outbreak in nine hospitalized South Korean patients; 12 non-duplicate multi-drug resistant ESBL-producing P. mirabilis strains were isolated. Bacteria were most frequently recovered from the respiratory tract (n=7), urine (n=3), and wounds (n=2). Such a trend is a matter of major concern, as P. mirabilis is a common cause of human infections (accounting for approximately 3% of all nosocomial infections), and ESBL-producing P. mirabilis strains are usually resistant to several antimicrobial agents and can be difficult to eliminate10. The organism is commonly resistant to ceftazidime, cefepime, and fluoroquinolones6. Thus, such antibiotics should not be used to treat infections with ESBL-producing P. mirabilis; carbapenems should be the drugs of choice7.
Pulmonary pneumatoceles occur as a complication of acute pneumonia, but are almost always transient. The lesions generally resolve spontaneously and completely, without sequelae134. The precise pathogenesis of a pneumatocele remains unclear. In one hypothesis, irritation and inflammation of a small bronchiole is thought to trigger formation of a mucus flap, which alternately opens and closes the bronchiolar orifice, effectively acting as a check-valve13. After resolution of the mucus check-valve by mucolytics and antibiotics, the stretched alveoli can be recovery13. Invasive intervensions may be considered in cases of tension pneumatocele, pneumothorax and infected pneumatocele occur14.
Pneumatoceles caused by ESBL-producing P. mirabilis pneumonia have not been previously described in the English-language literature. To the best of our knowledge, this is the first such report. The pneumatocele was initially diagnosed via chest CT, but no complication was evident. Multidrug-resistant ESBL-positive P. mirabilis was isolated, and the patient successfully treated with ertapenem for 4 weeks, leading to complete recovery.
In conclusion, respiratory physicians should be aware that pneumatoceles can develop in immunocompromised patients with pneumonia caused by uncommon pathogens such as ESBL-producing P. mirabilis. Monitoring of multidrugresistant strains is needed and physicians should make conscientious efforts to identify organisms causing unusual lung infections.
Notes
Conflicts of Interest: No potential conflict of interest relevant to this article was reported.