 |
 |
| Tuberc Respir Dis > Volume 78(4); 2015 > Article |
|
Abstract
Background
The adverse effects of the phosphodiesterase-4 inhibitor roflumilast, appear to be more frequent in clinical practice than what was observed in chronic obstructive pulmonary disease (COPD) clinical trials. Thus, we designed this study to determine whether adverse effects could be reduced by starting roflumilast at half the dose, and then increasing a few weeks later to 500 ┬Ąg daily.
Methods
We retrospectively investigated 85 patients with COPD who had taken either 500 ┬Ąg roflumilast, or a starting dose of 250 ┬Ąg and then increased to 500 ┬Ąg. We analyzed all adverse events and assessed differences between patients who continued taking the drug after dose escalation and those who had stopped.
Results
Adverse events were reported by 22 of the 85 patients (25.9%). The most common adverse event was diarrhea (10.6%). Of the 52 patients who had increased from a starting dose of 250 ┬Ąg roflumilast to 500 ┬Ąg, 43 (82.7%) successfully maintained the 500 ┬Ąg roflumilast dose. No difference in factors likely to affect the risk of adverse effects, was detected between the dose-escalated and the discontinued groups. Of the 26 patients who started with the 500 ┬Ąg roflumilast regimen, seven (26.9%) discontinued because of adverse effects. There was no statistically significant difference in discontinuation rate between the dose-escalated and the control groups (p=0.22).
Conclusion
Escalating the roflumilast dose may reduce treatment-related adverse effects and improve tolerance to the full dose. This study suggests that the dose-escalated regimen reduced the rate of discontinuation. However, longer-term and larger-scale studies are needed to support the full benefit of a dose escalation strategy.
Chronic obstructive pulmonary disease (COPD) is a leading cause of morbidity and mortality in adults worldwide. COPD is characterized by progressive airflow obstruction that is not fully reversible and an abnormal lung inflammatory response to noxious particles or gases. Obstructed airflow in patients with COPD results in chronic inflammation and structural changes in the small airways and lung parenchyma1.
Inflammation in COPD is characterized by increased numbers of neutrophils, macrophages, and cytotoxic T cells, as well as by the presence of several inflammatory mediators, such as cytokines, chemokines, and growth factors2,3. The search for an anti-inflammatory treatment for COPD is now focusing on inhibitors of phosphodiesterase (PDE) 4, the major cAMP hydrolase in inflammatory cells4. Roflumilast (3-cyclopropylmethoxy-4-difluoromethoxy-N-[3,5-dichloropyrid-4-yl] benzamide) is a PDE-4 targeted inhibitor of T-cell proliferation, cytokine production, and cell infiltration into the lungs. This agent is given orally once daily and is recommended for patients with COPD and severe airflow limitations (postbronchodilator forced expiratory velocity in 1 second/forced vital capacity [FEV1/FVC] <0.7; FEV1 <50%), chronic bronchitis symptoms, and a history of exacerbation not controlled by long-acting bronchodilators5,6.
The side effects of roflumilast detected in clinical studies include gastrointestinal difficulties, such as nausea, vomiting, and diarrhea, that resolve within a few weeks of continued treatment. However, adverse events appear more frequently in clinical practice than was observed in clinical trials. The frequency and severity of gastrointestinal adverse events from roflumilast are dose-dependent3. Therefore, the focus of this study is to explore whether the incidence of adverse effects can be reduced by an alternative dosing regimen.
The development of tolerance to roflumilast has not been thoroughly studied. The frequency of adverse events in clinical trials in patients using 250 ┬Ąg roflumilast was less than half that of those administered 500 ┬Ąg roflumilast7. Although adverse events may make roflumilast intolerable for up to 15% of subjects treated, most patients who develop severe adverse events do not interrupt their treatment, suggesting that the severity of the adverse events may be limited and that tolerance may develop8. One way to prevent initial adverse events is to progressively increase the initial drug dose over time9. We observed the side effects and tolerance of patients by prescribing 250 ┬Ąg oral roflumilast once daily, then stepping up to the full dose of 500 ┬Ąg after several weeks.
We retrospectively investigated patients with severe and very severe COPD, post-bronchodilator FEV1 <50% of the predicted value, post-bronchodilator FEV1/FVC ratio <70%, as defined by the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines, and a history of chronic bronchitis symptoms. We selected patients who initially took 250 ┬Ąg roflumilast and then increased to the full dose of 500 ┬Ąg after several weeks (Figure 1) and 500 ┬Ąg of roflumilast and compared them with the group in which the patients took 500 ┬Ąg of roflumilast throughout the period of the study. The two groups were compared using the chi-squared test. We also analyzed all adverse events reported during the lower dose (roflumilast 250 ┬Ąg) phase, whether patients continued taking the drug or stopped, and differences between these two groups. Statistical analyses were performed using the SPSS version 18.0 (SPSS Inc., Chicago, IL, USA). The Mann-Whitney U test was used to compare continuous variables, and Fisher's exact test was used to compare categorical variables. A p-value of <0.05 was considered to indicate statistical significant. All patients gave informed consent for this clinical trial. This study was undertaken from January 2013 to December 2014 at Gachon University Gil Medical Center.
Patients' baseline characteristics are shown in Table 1. A total of 59 patients who initially took 250 ┬Ąg roflumilast and then increased to 500 ┬Ąg were sequentially recruited for the study. All dose-escalated patients were male (mean age, 69.3┬▒8.2 years) and had been diagnosed with severe-to-very severe COPD (GOLD stages III and IV). The mean body mass index (BMI) of the patients was 20.9┬▒3.4 kg/m2, the mean height was 164.1┬▒6.5 cm, and the mean weight was 56.4┬▒9.8 kg. Twenty-nine patients (49.2%) used an inhaled corticosteroid (ICS), a long-acting beta agonist, and a long-acting muscarinic antagonist. In total, 26 patients who took 500 ┬Ąg were recruited for the control group (mean age, 71.9┬▒9.5 years). Of them, 21 patients (80%) were males. The mean BMI of control group was 20.1┬▒3.5 kg/m2, the mean height was 162.4┬▒8.9 cm, and the mean weight was 52.6┬▒8.6 kg. The two groups were significantly different in gender, underlying diseases, smoking status, and medications.
The discontinuation rate in the dose-escalated group (n=52), who initially took 250 ┬Ąg followed by 500 ┬Ąg of roflumilast was nine patients (17.3%). The number of patients who discontinued roflumilast in the control group (n=26) who initially took 500 ┬Ąg was seven patients (26.9%). The two groups did not differ significantly in terms of the ratio of failure (p=0.22).
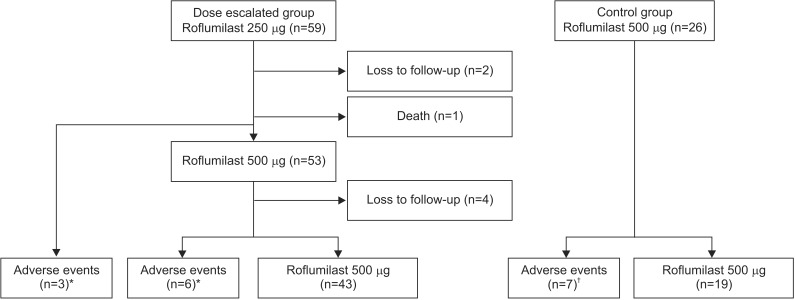
Six of the 59 patients given 250 ┬Ąg roflumilast did not increase to the approved dose of 500 ┬Ąg due to adverse effects (3 patients), from death due to acute exacerbation of COPD (1 patient), or loss to follow-up (2 patients). Fifty-three patients increased to 500 ┬Ąg roflumilast after 1 week to 3 months (mean, 81.6┬▒74.3; minimum, 6 days). Consequently, 43 of the 53 patients increased the roflumilast dose to 500 ┬Ąg with tolerable side effects. However, six patients needed to decrease the dose back to 250 ┬Ąg due to intolerable side effects. Four patients were not followed up (Figure 1). We analyzed differences between patients who continued after dose escalation (n=43) and those who stopped (n=9). No significant difference was found in body surface area, height, body weight, age, BMI, underlying diseases, smoking status, or combined medications between the two groups (Table 2).
Of the 59 patients in the dose-escalated group, adverse events occurred in 14 (23.7%). The most common adverse events were diarrhea (6 patients), weight loss (5 patients), poor oral intake (4 patients), nausea (3 patients), headache (2 patients), skin rash (2 patients), and lower leg pain (2 patients). Of the 26 patients in the control group, adverse events occurred in seven (26.9%). Adverse events were diarrhea (3 patients), nausea (2 patients), poor oral intake (1 patient), and headache (1 patient) (Table 3).
Three patients were unable to maintain the full dose due to loss to follow-up or death. Of the remaining 56 patients, 53 (95%) continued to take 250 ┬Ąg roflumilast, and three could not maintain the full dose due to adverse events, such as diarrhea, poor oral intake, and rash. Of the 53 patients who attempted to increase to 500 ┬Ąg roflumilast, 43 were able to continue taking the 500 ┬Ąg dose. Four of these patients who were not followed up; the remaining six could not maintain the 500 ┬Ąg dose due to adverse events, such as diarrhea, nausea, weight loss, poor oral intake, and skin rash. Although seven patients who increased to 500 ┬Ąg roflumilast complained of adverse events, including diarrhea (3 patients), weight loss (2 patients), leg pain (2 patients), headache (1 patient), and nausea (1 patient), they tolerated the 500 ┬Ąg dose and took it continuously.
PDE-4 inhibitors are a novel drug class that has emerged to treat COPD. Roflumilast is the first member of this class to be licensed and is effective when used concomitantly with a long-acting beta-2 agonist, a long-acting anticholinergic, and ICS10. Roflumilast has been considered safe and well tolerated during long-term administration in clinical studies. Patients with COPD were randomized to placebo, 250 ┬Ąg, or 500 ┬Ąg roflumilast once daily for 24 weeks in an early dose-ranging study (RECORD), which found that the lower roflumilast dose was generally associated with fewer adverse effects than the higher dose. Serious adverse events were reported by 10% of patients taking 500 ┬Ąg roflumilast, 7% of those taking 250 ┬Ąg, and in 8% of those taking placebo. The most common adverse effect was diarrhea, which had a much higher frequency in patients taking 500 ┬Ąg than the lower dose during the first 4 weeks. The frequency of diarrhea in patients taking both roflumilast doses was comparable to that for the placebo by week 132. Other studies have shown similar frequencies of adverse effects in response to roflumilast and placebo (67.2% vs. 62.8%, respectively) and gastrointestinal difficulties were the most frequent (higher rates of diarrhea, nausea, vomiting, and decreased appetite), as well as the main reason for terminating the study early7,10. Discontinuation due to adverse events in the 12-month M2-124 and M2-125 studies was more common in patients taking roflumilast (14%) than those taking placebo (11%)10,11.
In contrast to the discontinuation rates in these pivotal studies3, clinicians experience frequent adverse events in clinical practice. Alternative dosing strategies including dose escalation have not been clinically investigated. However, population pharmacokinetic/pharmacodynamics modeling3 found that an alternative dose regimen, i.e., 250 ┬Ąg once daily and the approved dose (500 ┬Ąg) every other day regimens had minimal impact on efficacy and may reduce the risk of related adverse effects. Similarly, the frequencies of diarrhea, nausea, and headache were about two-fold lower in patients taking 250 ┬Ąg roflumilast once daily than in those taking the approved 500 ┬Ąg once daily regimen3. In the present study, of the 53 patients who increased to 500 ┬Ąg roflumilast, 38 suffered no adverse effects. Seven patients complained of diarrhea but it was not severe enough to discontinue 500 ┬Ąg roflumilast. Patients who are aware of the transient nature of the adverse effects associated with roflumilast may have been motivated to continue taking the drug until the adverse effects resolved. No significant difference was observed between those who continued taking the drug and those who stopped. However, the observed p-value for body surface area was 0.06 between the two groups, suggesting a possible correlation.
In our study, we showed that the rate (17.3%) of discontinuation in the dose-escalated group was lower than that rate (26.9%) in the control group. Roflumilast was well tolerated in patients who started taking 250 ┬Ąg, and a decrease in the number of adverse events was observed when they increased to 500 ┬Ąg. However, the difference between the dose-escalated and control groups was not statistically significant (p=0.22). This is due in part to the small number of patients analyzed, and the significant differences in gender, underlying disease, smoking history, and medications between the two groups. In conclusion, we did not find a statistically significant difference with the dose-escalated regimen in terms of maintaining 500 ┬Ąg roflumilast, but showed a numerical decrease in treatment-related adverse effects leading to discontinuation of roflumilast in the dose-escalated group. Thus, longer-term and larger-scale studies are needed to fully assess the benefit of a roflumilast dose-escalation strategy.
References
1. Hogg JC. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet 2004;364:709-721. PMID: 15325838.


2. Rabe KF, Bateman ED, O'Donnell D, Witte S, Bredenbroker D, Bethke TD. Roflumilast: an oral anti-inflammatory treatment for chronic obstructive pulmonary disease: a randomised controlled trial. Lancet 2005;366:563-571. PMID: 16099292.


3. Lahu G, Facius A. Application of population pharmacokinetic modeling to explore the impact of alternative roflumilast dosing regimens on tolerability. Int J Clin Pharmacol Ther 2013;51:832-836. PMID: 24152603.


4. Torphy TJ, Barnette MS, Underwood DC, Griswold DE, Christensen SB, Murdoch RD, et al. Ariflo (SB 207499), a second generation phosphodiesterase 4 inhibitor for the treatment of asthma and COPD: from concept to clinic. Pulm Pharmacol Ther 1999;12:131-135. PMID: 10373396.


5. Bundschuh DS, Eltze M, Barsig J, Wollin L, Hatzelmann A, Beume R. In vivo efficacy in airway disease models of roflumilast, a novel orally active PDE4 inhibitor. J Pharmacol Exp Ther 2001;297:280-290. PMID: 11259555.

6. Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013;187:347-365. PMID: 22878278.


7. Michalski JM, Golden G, Ikari J, Rennard SI. PDE4: a novel target in the treatment of chronic obstructive pulmonary disease. Clin Pharmacol Ther 2012;91:134-142. PMID: 22130119.


8. Beghe B, Rabe KF, Fabbri LM. Phosphodiesterase-4 inhibitor therapy for lung diseases. Am J Respir Crit Care Med 2013;188:271-278. PMID: 23656508.


9. Giembycz MA, Newton R. Harnessing the clinical efficacy of phosphodiesterase 4 inhibitors in inflammatory lung diseases: dual-selective phosphodiesterase inhibitors and novel combination therapies. Handb Exp Pharmacol 2011;(204):415-446. PMID: 21695651.


10. Rabe KF. Update on roflumilast, a phosphodiesterase 4 inhibitor for the treatment of chronic obstructive pulmonary disease. Br J Pharmacol 2011;163:53-67. PMID: 21232047.



11. Calverley PM, Rabe KF, Goehring UM, Kristiansen S, Fabbri LM, Martinez FJ, et al. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet 2009;374:685-694. PMID: 19716960.


Figure┬Ā1
Follow-up flow chart for patients on the roflumilast dose escalation regimen and control group. *Dose escalated failed. ŌĆĀDiscontinued.
Table┬Ā1
Baseline patient characteristics
Table┬Ā2
Comparison of patients who continued medication to those who discontinued, in the dose escalated group
- TOOLS
-
METRICS

- Related articles
-
What Single Cell RNA Sequencing Has Taught Us about Chronic Obstructive Pulmonary Disease


 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Print
Print Download Citation
Download Citation



