Lung Disease Caused by Mycobacterium malmoense in an Immunocompetent Patient
Article information
Abstract
Mycobacterium malmoense is a very rare cause of lung disease in South Korea. We reported the first case of lung disease caused by M. malmoense in an immunocompetent patient. The patient was successfully treated with a 14-month course of antibiotics.
Introduction
Because non-tuberculous mycobacteria (NTM) are increasingly recognized as human pathogens, NTM disease is an emerging public health threat. Mycobacterium avium complex is the most common lung disease-causing NTM pathogen in South Korea; the second most common is M. abscessus complex12. Although M. malmoense, a slow-growing non-photochromogenic mycobacterium, is the second most common cause of NTM lung disease in northern Europe3, there have been no reported cases in South Korea. Here, we report the first case of M. malmoense pulmonary infection in an immunocompetent patient.
Case Report
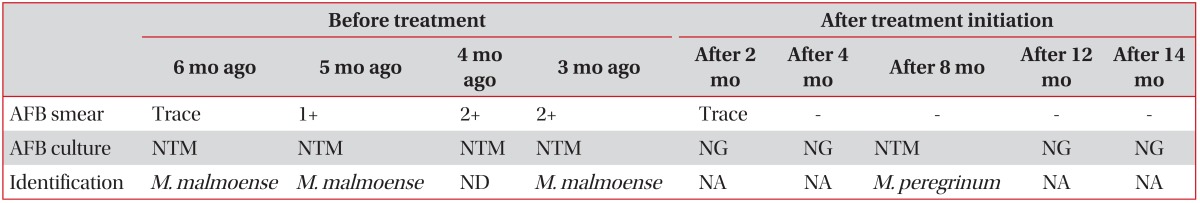
A 66-year-old man visited our hospital, Asan Medical Center, due to a cough persisting for 5 months. Seven years earlier, he had been treated for pan-susceptible pulmonary tuberculosis (TB), which lasted for 9 months. This treatment was successful but a chest X-ray (CXR) at the time of treatment completion showed parenchymal destructive lesions, with fibrotic changes in the right upper lung field (Figure 1). He also suffered from a mild chronic cough. He remained relatively well thereafter. A CXR taken about 1 year earlier showed no significant changes in the TB-destroyed lung. However, his cough became aggravated 6 months prior to presentation at our clinic.

Chest X-ray taken 7 years earlier showing parenchymal destruction, fibrotic lesions, and bullous changes in the right upper lung field caused by a previous episode of pulmonary tuberculosis.
Physical examination revealed that the patient was alert and not in distress. His vital signs were as follows: temperature, 35.6℃; blood pressure, 123/78 mm Hg; pulse, 63 beats per minute (with a regular rhythm); and respiratory rate, 20 breaths per minute. Auscultation revealed decreased breath sounds in the right upper anterior chest. Blood tests showed a white blood cell count of 7,500/mm3 (67% neutrophils); hemoglobin, 12.6 g/dL; and a platelet count of 268,000/mm3. Routine chemical laboratory data were all within the normal ranges. The patient was negative for antibodies to human immunodeficiency virus.
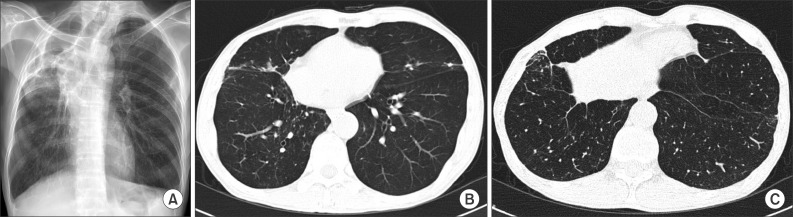
Compared with the CXR taken 6 years earlier, his CXR showed increased infiltration of the right middle lung field, with small nodular densities (Figure 2A). A chest computed tomography (CT) scan revealed a newly developed segmental centrilobular nodular lesion in the right lung (Figure 2B, C). Smears and cultures of multiple sputum specimens were positive for acid-fast bacilli, which a GenoType Mycobacterium Assay (Hain Diagnostika, Nehren, Germany) identified as M. malmoense (Table 1).
(A) Chest X-ray showing newly developed infiltrative lesions in the right middle lung area. (B) Low-dose chest computed tomography image showing newly developed segmental centrilobular nodules in the right lung. (C) Low-dose chest computed tomography image showing multifocal patches of consolidation and nodules in the right lung.
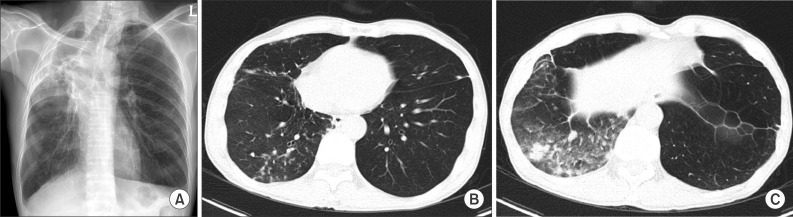
Based on these clinical and laboratory data, the patient was given a diagnosis of lung disease caused by M. malmoense, according to the American Thoracic Society (ATS) criteria. M. malmoense was subjected to several rounds of drug susceptibility testing; however, all failed due to contamination. The patient was treated with oral isoniazid, rifampicin, ethambutol, moxifloxacin, and clarithromycin, and his symptoms improved rapidly. Sputum culture conversion was observed after 2 months. Due to the improved clinical course, moxifloxacin was stopped 8 months after initiation. Also, we had to discontinue ethambutol after 10 months due to the onset of optic neuropathy. Treatment was completed 12 months after achieving sputum culture conversion (i.e., treatment lasted 14 months in total). A CXR and CT scan taken 12 months after the start of treatment showed an improvement in the lung lesions (Figure 3). The patient received regular follow-up for 3 months after treatment completion with no relapse of M. malmoense infection.
Discussion
Although the incidence of NTM lung disease is rising, M. malmoense is a rare lung disease-causing pathogen in South Korea. Here, we describe a very rare case of M. malmoense infection in an immunocompetent Korean male patient. To the best of our knowledge, this is the first reported case of lung disease caused by M. malmoense in South Korea. The patient was successfully treated with antibiotics.
There is marked geographic variability in the incidence of M. malmoense disease. For example, M. malmoense is the second most frequently isolated NTM in northern Europe4, but is rare in North America5. Accordingly, clinically relevant infections were identified in 70% of cases in northern Europe4, but only in 10% of cases in North America5. The reason for this discrepancy is unclear, although environmental factors are one possible explanation. Disease caused by M. malmoense has not previously been reported in South Korea. Moreover, Ryoo et al.6 reported previously that they failed to isolate M. malmoense from 17,915 NTM organisms in their reference laboratory at a single South Korean institution over a 14-year period. Although our current case is the first documented patient with M. malmoense lung disease in South Korea, it should be noted that we only identified the pathogen using a commercial line probe assay kit. We could not perform sequencing analysis because we were unable to obtain NTM isolates of our case, since they had been disposed of long before.
NTM lung disease develops more frequently in patients with pre-existing lung disease; this is because regional factors within the damaged areas impair the host immune response, disrupt normal defense mechanisms, influence the relationship between ventilation and perfusion, and distort the lung architecture7. Hoefsloot et al.3 reported that the majority of patients that contracted M. malmoense lung disease in the Netherlands were male and had pre-existing pulmonary disease (most often chronic obstructive pulmonary disease). Our current case also had a pre-existing pulmonary condition: a TB-destroyed lung, although M. malmoense disease mainly affected the normal lung parenchyma.
Approximately two thirds of patients with M. malmoense lung disease show a good therapeutic response34. ATS guidelines recommend combination therapy with isoniazid, rifampin, and ethambutol, either with or without quinolones and macrolides8. However, a British Trial Society (BTS) trial showed that adjuvant clarithromycin or ciprofloxacin during a 24-month regimen of rifampin and ethambutol had no additional benefit for the treatment of M. malmoense pulmonary disease9. In addition, another BTS trial reported that a 2-year regimen comprising rifampin and ethambutol was better tolerated than rifampin and ethambutol combined with isoniazid and had a similar outcome10. However, we successfully treated our current patient with a combination of isoniazid, moxifloxacin, and clarithromycin in addition to rifampin and ethambutol over a short period of time. It may be that, in this case, the addition of moxifloxacin resulted in a successful treatment outcome within a short time. A previous study revealed that a combination of moxifloxacin, clarithromycin, and ethambutol showed therapeutic efficacy against M. avium11. Moreover, moxifloxacin is more effective against mycobacterial disease than older quinolone112. However, although moxifloxacin has a favorable therapeutic effect against M. avium, it is unclear whether it also has a favorable effect against M. malmoense because no studies have examined this issue. Further studies are needed to fully understand the role of moxifloxacin in the treatment of lung disease caused by M. malmoense.
In summary, we report the first case of pulmonary infection in South Korea caused by M. malmoense, which was successfully treated with antibiotics.
Notes
Conflicts of Interest: No potential conflict of interest relevant to this article was reported.