 |
 |
| Tuberc Respir Dis > Volume 78(3); 2015 > Article |
|
Abstract
Severe sepsis and septic shock is a life-threatening disease. It is combined with multi-organ failure. In the past decade, early goal directed therapy has been proposed as an effective treatment strategy for better outcome. Recent epidemiologic studies showed that the outcome of sepsis has been improved with the introduction of early goal directed therapy. However, it is unclear which elements of early goal directed therapy contributed to the better outcome. Recent prospective and randomized trials suggested that some elements of early goal directed therapy did not have any effect on the outcome benefit. In this paper, recent articles about early goal directed therapy will be reviewed and the effectiveness of individual elements of early goal directed therapy will be discussed.
During the past 30 years, the incidence of sepsis has been increased and the related mortality rate amounts to 50 percent in the most severe cases1,2. In 2001, Rivers et al.3 suggested early goal directed therapy (EGDT) improved the survival of severe sepsis and septic shock. EGDT refers to a treatment bundle including early intensive fluid administration using physiologic targets to guide resuscitation within first six hours in severe sepsis and septic shock. Thereafter, EGDT has widely accepted in clinical practice; however, there is conflicting evidences regarding the effectiveness of individual resuscitation elements and targets3,4,5. In order to solve this controversy, well designed, large scaled, randomized trial has been published. Recently, ProCESS study5 and ARISE study4 reported no mortality benefit with EGDT-based therapies. However, there are recent evidences supporting the benefit of EGDT6,7,8,9. In this review, therefore, debates on EGDT and recent evidences regarding sepsis treatments will be discussed.
Sepsis is defined as a systemic inflammatory response syndrome caused by bacterial infection10. According to severity of sepsis, it is classified into sepsis, severe sepsis and septic shock. Severe sepsis is defined as the state which is combined with sepsis induced organ failure or tissue hypoperfusion. Septic shock is defined as sepsis-induced hypotension persisting despite adequate fluid administration.
Since the 1970s, the incidence of sepsis has been increasing every year in the United States. Hospitalization due to sepsis has been increased by 7.9% annually since 2000 and sepsis has become the most common causes of hospitalization in the sixth1. This increase in incidence is thought to be related to the increase in immunocompromised host, the elderly and multi-drug resistant strains11,12,13,14. In contrast to the increase in the incidence of sepsis, mortality has decreased over the past decade7,9,15,16. One of these studies showed that sepsis mortality in 2012 was 14.2% in severe sepsis and 22.0% in septic shock, respectively9. Notably in this study, mortality of sepsis was reduced down from 2000 to 2012 even after adjusting for the effects of increased incidence, co-morbidities, age and severity. This suggested that the outcome of sepsis has been improved during a few past decades regardless of increased early detection of sepsis.
The causes of recent improvement of sepsis outcome might be multi-factorial. The Surviving Sepsis Campaign (SSC) activities were particularly noteworthy and helpful in the sepsis management. The SSC established and updated the sepsis guideline every four years since 200410,17,18. A recent study presented the evidences that the activities of the SSC or EGDT was associated with improved outcome8. In 2009, MOSAICS study assessed sepsis treatment status of Asian intensive care units (ICUs) and hospitals in terms of the compliance of the SSC guideline6. In this study, the mortality of severe sepsis was 44.5% in all included countries and 38.7% in the high income countries including Korea. However, the EGDT bundle compliance was quite low (10%) even in the high income country and was related to poor outcome. From this study, the mortality of sepsis in Korea is still high comparing to Western countries and this gap was presumed to be related with low EGDT bundle compliance (10%).
Since the introduction of EGDT in 2001, the importance of early treatment for tissue oxygenation (tissue oxygenation) in sepsis is emphasized to improve the prognosis. In particular, treatment goals for improving tissue oxygenation, such as central venous pressure (CVP), mean arterial blood pressure (MAP), central venous oxygen saturation (ScvO2), and blood lactate concentration were achieved as a protocolized bundle within 6 hour and effort to raise resuscitation bundle compliance was emphasized (Table 1). However, the evidences of each of the goals and the resuscitation element are controversial and not validated. Recently, many studies about these controversies have been published. So, these controversies and potential guidance about sepsis treatment will be discussed afterward.
As mentioned earlier, recent evidences showed that the resuscitation bundle including EGDT improved the prognosis of sepsis6,8,9. In a multi-center observational study of United States, as SSC guideline compliance increased by 68.5%, in-hospital mortality was reduced by 59%8. Especially if initial resuscitation element which is performed within 3 hours is well done, this prevented the worsening severity of sepsis and the requirements for further resuscitation factors, such as vasopressor, transfusion of red blood cells, steroid use, and low tidal ventilation in many cases. Therefore, this can warrant once again the importance of early implementation of the sepsis bundle.
Recently, ProCESS study suggested the contradictory results (relative risk with protocol-based therapy vs. usual care, 1.04; 95% confidence interval [CI], 0.82-1.31; p=0.83 and relative risk with protocol-based EGDT vs. protocol-based standard therapy, 1.15; 95% CI, 0.88-1.51; p=0.31) from previous data and raised questions about the effects of sepsis resuscitation bundle. Additionally, ARISE study also demonstrated that EGDT did not reduce all-cause mortality in early septic shock4. However, in these studies, the mortality of both treatment group was relatively low (18.6%-21.0%) comparing the outcome of past studies. In addition, both treatment group were early diagnosed with severe sepsis or septic shock and thereafter received the substantial amount (1-2 L) of crystalloid. The proportion of the early use of antibiotics was quite high (75%). These facts imply that 3hr resuscitation of the SSC guideline was basically achieved in both treatment groups. Therefore, combining the results of recent epidemiologic study, the main causes of the improved outcome is the 3 hour resuscitation element of EGDT, not the goal-directed therapy at ScvO2, CVP and Hct above 30%.
The guideline recommends a protocolized, quantitive resuscitation if sepsis induced tissue hypoperfusion (blood lactate ≥4 mmol/L or if hypotension is persisting despite bolus supply of fluid). However, there is a controversy about the optimal volume and types of resuscitative fluid. Here, the evidences about this controversy will be reviewed.
In the Saline versus Albumin Fluid Evaluation (SAFE) trial19, 6,997 critically ill patients were randomly assigned to receive 4% albumin solution or normal saline for up to 28 days. There were no differences between two groups for mortality. In a subgroup with severe sepsis (18% of the total group), there was a trend toward decreased mortality in the albumin group (risk ratio, 0.93; 95% CI, 0.61-1.41; p=0.09). From this result, the SAFE Study Investigators evaluated the safety of albumin as a resuscitative fluid in severe sepsis20. As a result, they concluded that administration of albumin compared to saline did not impair renal or other organ function (18.7% vs. 18.2%; p=0.98) and may have decreased the risk of death (adjusted odds ratio [OR], 0.71; 95% CI, 0.52-0.97; p=0.03).
Previous two studies of the SAFE Study Investigators suggested the evidence for the safety of albumin fluid in severe sepsis but the survival benefit against crystalloid fluid was not validated. To confirm this topic, the SAFE Study Investigators recently published another multicenter open-label randomized trial of patients with severe sepsis or septic shock21. In this study, 1,818 patients with severe sepsis assigned to receive either 20% albumin and crystalloid solution or crystalloid solution alone. However, albumin replacement in addition to crystalloids did not improve the 28-day mortality and any other end points including 90-day mortality, the number and the degree of organ dysfunction, and hospital stays. Therefore, crystalloid is the first choice of resuscitative fluid in severe sepsis or septic shock. Albumin is safe and useful in intravascular volume expansion consistent with the observations from previous studies19,20,21.
Among the colloid fluids, hydroxyethyl starch (HES) was widely used for fluid resuscitation in ICUs. However, in a multicenter study, patients with severe sepsis assigned to fluid resuscitation with HES 130/0.42 had an increased risk of death at day 90 and were more likely to require renal-replacement therapy22. Thereafter, the SSC decided to ban HES for patients with sepsis10.
The guideline recommends a target blood pressure (BP) of 65 mm Hg or more and also encouraged to modify a target BP depending on the underlying atherosclerosis or hypertension. However, the strength of the evidence about target BP is weak and has not been validated fully23,24. In 2014, SEPSISPAM study has confirmed that the recommendation of the guideline is proper25. In this multicenter, open-label trial, a total of 776 patients were assigned to the low target group (MAP, 65-70 mm Hg) and high target group (MAP, 80-85 mm Hg) between the 28th days. However, there was no difference in mortality rate at 28 day (hazard ratio in the high-target group, 1.07; 95% CI, 0.84-1.38; p=0.57). Patients with a higher MAP had a greater incidence of atrial fibrillation (6.7% vs. 2.8%, p=0.02), otherwise in patients with a history of chronic hyptenstion, a higher MAP reduced the rate of renal replacement therapy (42.2% vs. 31.7%, p=0.04). Therefore, target BP above 65 mm Hg or more is recommended in sepsis or septic shock as mentioned above.
The guideline recommended the monitoring ScvO2 and lactate as a goal for resucitation. However, In ProCESS and ARISE study, goal directed resuscitation targeting ScvO2 above 70% or more showed no mortality benefit. ScvO2 is one of useful index for tissue hypoperfusion, however, the effectiveness as single goal of sepsis treatment is questionable. Lactate has been suggested as an alternative goal and monitoring index for resuscitation in severe sepsis or septic shock. In a randomized clinical trial26, lactate clearance ([(Initial lactate-Lactate >2 hours later)/Initial lactate]×100) showed non-inferiority in mortality for ScvO2 as a goal of resuscitation of septic shock (23% vs. 17%; 95% CI, 11%-24%). In another study27, lactate normalization within 6 hours was the strongest predictor of survival (adjusted OR, 5.2; 95% CI, 1.7-15.8) in severe sepsis. From these results, serial measurement of lactate is expected to be used as an alternative to ScvO2 if central venous insertion is not available. However, it should be considered that lactate is not sensitive to reflect the change of tissue perfusion after restoration of perfusion28.
The SSC responded to the result of the recent two clinical trials, ProCESS and ARISE study as following; the monitoring of ScvO2 and CVP is not routinely required in all patients with severe sepsis or septic shock. However, initial 3-hour bundle of the guideline will not be affected by these trials and SSC bundle did not confer any harm result even though did not confer any benefit. Therefore, keeping the current guideline is intact until evidences are growing enough.
From these evidences, it is obvious that ScvO2 and CVP is not a best goal for resuscitation in severe sepsis and septic shock. However, protocol directed therapy requires the use of a physiologic endpoint to guide fluid management. ProCESS and ARISE study showed that traditional physiologic targets using MAP, urine output were not inferior to EGDT targets (ScvO2, CVP, MAP, and urine output). Therefore, a protocolized therapy is basically targeted to MAP and urine output and additional targets including ScvO2 and CVP can be used according to an individualized clinical situation.
Vassopressor is recommended if hypotension is ongoing despite adequate fluid resuscitation. Traditionally, norepinephrine and dopamine is commonly used, but recent trials comparing two vasopressors favor the use of norepinephrine. In 2010, SOAP II study showed that the comparison of two vasopressor in sepsis found no difference in mortality (OR with dopamine, 1.17; 95% CI, 0.97-1.42; p=0.10)29. However, the use of dopamine was associated with more frequent arrhythmic events. Also, the subgroup of cardiogenic shock was associate with higher mortality in the dopamine group (p=0.03). In addition, a meta-analysis showed that dopamine administration is associated with greater mortality and a higher incidence of arrhythmic events compared to norepinephrine administration30. Therefore, norepinephrine is a reasonable first choice in the patients with severe sepsis and septic shock.
Red cell transfusion was basically reserved for patients with a hemoglobin level below 7 g/dL or less. Data from randomized trial based on EGDT showed conflicting results3,4,5. Recent two trials, such as ProCESS and ARISE study showed that red blood cell (RBC) transfusion did not showed any benefits as part of the protocolized therapy. One multicenter randomized trial was conducted to solve this controversy33. The patients with septic shock were assigned to lower and higher threshold groups defined as hemoglobin ≤7.0 g/dL and hemoglobin ≤9.0 g/dL, respectively. The mortality at 90 days and rates of ischemic events and use of life support were similar among both groups. Therefore, RBC transfusion in patients below hemoglobin 7.0 g/dL or less is the consensus for severe sepsis and septic shock except if combined hemorrhagic shock or myocardial ischemia.
Since the Rivers' landmark study in 2001, EGDT has been a backbone of sepsis treatment and led to the SSC. Many epidemiologic studies support the positive effect of the past decade's treatment strategy on the outcome of sepsis. Nevertheless, controversy persists over the each element of resuscitation management or resuscitation goal. Recent two randomized trial with similar design with Rivers' study clarified the drawback of EGDT. As a result, we could identify that initial 3-hour resuscitation is still very important with early detection of the onset of severe sepsis and septic shock. Also, recent randomized trials (ProCESS & ARISE) confirmed that the recommendation of 2012 SSC guideline is effective in the resuscitation elements or goal including the threshold of MAP or transfusion. The other resuscitation goals including CVP, ScVO2 and lactate were not proven to be effective in patients with severe sepsis and septic shock. However, there is no evidence about the harm from keeping these resuscitation goals. Therefore, it is reasonable not to change the current clinical practice. Especially, early detection of severe sepsis and septic shock and 3-hour management bundle should be kept.
References
1. Elixhauser A, Friedman B, Stranges E. Septicemia in U.S. hospitals, 2009: statistical brief #122. Healthcare Cost and Utilization Project (HCUP) statistical briefs. Rockville: Agency for Health Care Policy and Research; 2006.
2. Sasse KC, Nauenberg E, Long A, Anton B, Tucker HJ, Hu TW. Long-term survival after intensive care unit admission with sepsis. Crit Care Med 1995;23:1040-1047. PMID: 7774214.


3. Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 2001;345:1368-1377. PMID: 11794169.


4. ARISE Investigators. ANZICS Clinical Trials Group. Peake SL, Delaney A, Bailey M, Bellomo R, et al. Goal-directed resuscitation for patients with early septic shock. N Engl J Med 2014;371:1496-1506. PMID: 25272316.


5. ProCESS Investigators. Yealy DM, Kellum JA, Huang DT, Barnato AE, Weissfeld LA, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med 2014;370:1683-1693. PMID: 24635773.



6. Phua J, Koh Y, Du B, Tang YQ, Divatia JV, Tan CC, et al. Management of severe sepsis in patients admitted to Asian intensive care units: prospective cohort study. BMJ 2011;342:d3245PMID: 21669950.



7. Lagu T, Rothberg MB, Shieh MS, Pekow PS, Steingrub JS, Lindenauer PK. Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Crit Care Med 2012;40:754-761. PMID: 21963582.


8. Miller RR 3rd, Dong L, Nelson NC, Brown SM, Kuttler KG, Probst DR, et al. Multicenter implementation of a severe sepsis and septic shock treatment bundle. Am J Respir Crit Care Med 2013;188:77-82. PMID: 23631750.



9. Kaukonen KM, Bailey M, Suzuki S, Pilcher D, Bellomo R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000-2012. JAMA 2014;311:1308-1316. PMID: 24638143.


10. Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 2013;41:580-637. PMID: 23353941.



11. Blanco J, Muriel-Bombin A, Sagredo V, Taboada F, Gandia F, Tamayo L, et al. Incidence, organ dysfunction and mortality in severe sepsis: a Spanish multicentre study. Crit Care 2008;12:R158PMID: 19091069.



12. Danai P, Martin GS. Epidemiology of sepsis: recent advances. Curr Infect Dis Rep 2005;7:329-334. PMID: 16107228.



13. Esper AM, Martin GS. Extending international sepsis epidemiology: the impact of organ dysfunction. Crit Care 2009;13:120PMID: 19291262.



14. Harrison DA, Welch CA, Eddleston JM. The epidemiology of severe sepsis in England, Wales and Northern Ireland, 1996 to 2004: secondary analysis of a high quality clinical database, the ICNARC Case Mix Programme Database. Crit Care 2006;10:R42PMID: 16542492.



15. Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 2003;348:1546-1554. PMID: 12700374.


16. Zimmerman JE, Kramer AA, Knaus WA. Changes in hospital mortality for United States intensive care unit admissions from 1988 to 2012. Crit Care 2013;17:R81PMID: 23622086.



17. Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med 2008;36:296-327. PMID: 18158437.


18. Dellinger RP, Carlet JM, Masur H, Gerlach H, Calandra T, Cohen J, et al. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med 2004;32:858-873. PMID: 15090974.


19. Finfer S, Bellomo R, Boyce N, French J, Myburgh J, Norton R, et al. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med 2004;350:2247-2256. PMID: 15163774.


20. SAFE Study Investigators. Finfer S, McEvoy S, Bellomo R, McArthur C, Myburgh J, et al. Impact of albumin compared to saline on organ function and mortality of patients with severe sepsis. Intensive Care Med 2011;37:86-96. PMID: 20924555.


21. Caironi P, Tognoni G, Masson S, Fumagalli R, Pesenti A, Romero M, et al. Albumin replacement in patients with severe sepsis or septic shock. N Engl J Med 2014;370:1412-1421. PMID: 24635772.


22. Perner A, Haase N, Guttormsen AB, Tenhunen J, Klemenzson G, Aneman A, et al. Hydroxyethyl starch 130/0.42 versus Ringer's acetate in severe sepsis. N Engl J Med 2012;367:124-134. PMID: 22738085.


23. LeDoux D, Astiz ME, Carpati CM, Rackow EC. Effects of perfusion pressure on tissue perfusion in septic shock. Crit Care Med 2000;28:2729-2732. PMID: 10966242.


24. Bourgoin A, Leone M, Delmas A, Garnier F, Albanese J, Martin C. Increasing mean arterial pressure in patients with septic shock: effects on oxygen variables and renal function. Crit Care Med 2005;33:780-786. PMID: 15818105.


25. Asfar P, Meziani F, Hamel JF, Grelon F, Megarbane B, Anguel N, et al. High versus low blood-pressure target in patients with septic shock. N Engl J Med 2014;370:1583-1593. PMID: 24635770.


26. Jones AE, Shapiro NI, Trzeciak S, Arnold RC, Claremont HA, Kline JA, et al. Lactate clearance vs central venous oxygen saturation as goals of early sepsis therapy: a randomized clinical trial. JAMA 2010;303:739-746. PMID: 20179283.



27. Puskarich MA, Trzeciak S, Shapiro NI, Albers AB, Heffner AC, Kline JA, et al. Whole blood lactate kinetics in patients undergoing quantitative resuscitation for severe sepsis and septic shock. Chest 2013;143:1548-1553. PMID: 23740148.


28. Forsythe SM, Schmidt GA. Sodium bicarbonate for the treatment of lactic acidosis. Chest 2000;117:260-267. PMID: 10631227.


29. De Backer D, Biston P, Devriendt J, Madl C, Chochrad D, Aldecoa C, et al. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med 2010;362:779-789. PMID: 20200382.


30. De Backer D, Aldecoa C, Njimi H, Vincent JL. Dopamine versus norepinephrine in the treatment of septic shock: a meta-analysis. Crit Care Med 2012;40:725-730. PMID: 22036860.


31. Hayes MA, Timmins AC, Yau EH, Palazzo M, Hinds CJ, Watson D. Elevation of systemic oxygen delivery in the treatment of critically ill patients. N Engl J Med 1994;330:1717-1722. PMID: 7993413.


32. Gattinoni L, Brazzi L, Pelosi P, Latini R, Tognoni G, Pesenti A, et al. A trial of goal-oriented hemodynamic therapy in critically ill patients. SvO2 Collaborative Group. N Engl J Med 1995;333:1025-1032. PMID: 7675044.


33. Holst LB, Haase N, Wetterslev J, Wernerman J, Guttormsen AB, Karlsson S, et al. Lower versus higher hemoglobin threshold for transfusion in septic shock. N Engl J Med 2014;371:1381-1391. PMID: 25270275.


Table 1
Surviving sepsis campaign care bundles
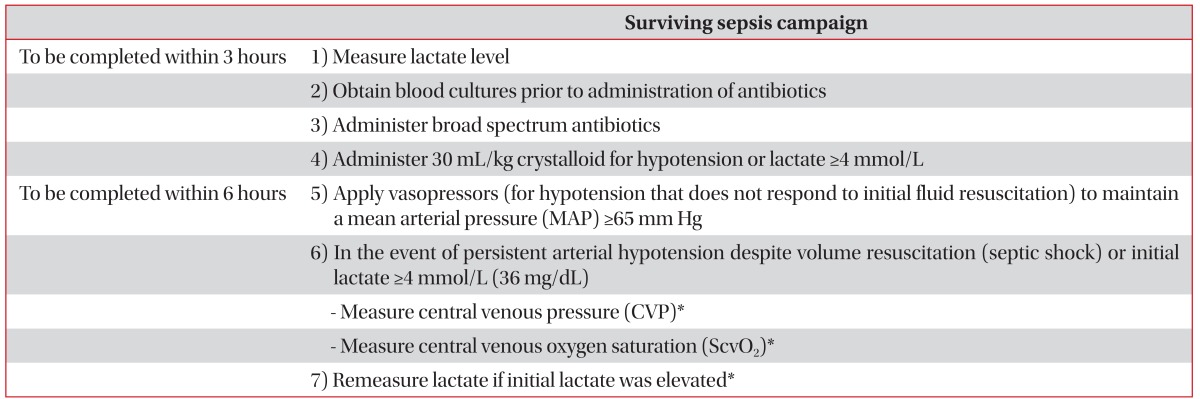
Adapted from Dellinger RP et al. Crit Care Med 2013;41:580-637, with permission of Wolters Kluwer Health, Inc.10.
*Targets for quantitative resuscitation included in the guidelines are CVP of ≥8 mm Hg, ScvO2 of ≥70%, and normalization of lactate.


 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Print
Print Download Citation
Download Citation




