 |
 |
| Tuberc Respir Dis > Volume 78(2); 2015 > Article |
|
Abstract
Solitary fibrous tumor of the pleura (SFTP) is a rare primary intrathoracic tumor that arises from mesenchymal tissue underlying the mesothelial layer of the pleura. It usually has an indolent clinical course. The hypoglycemia that accompanies SFTP was first described by Doege and Potter independently in 1930, hence the eponym Doege-Potter syndrome (DPS). The incidence of DPS is reported to be ~4%. In this report, we present a typical case of DPS that was cured through complete surgical resection.
Solitary fibrous tumor of the pleura (SFTP) is a rare, slow-growing tumor that accounts for about 5% of all pleural neoplasms1. About 80% of SFTPs are benign, and about 50% appear as an asymptomatic mass, which may be discovered in a routine chest X-ray2,3. Symptoms include cough and chest pain, fatigue, weight loss, dyspnea, and fever4. More rarely, hemoptysis, hypertrophic pulmonary osteoarthropathy, and hypoglycemia may occur2. Hypoglycemia occurs in 2%-4% of cases2. The hypoglycemia that accompanies SFTP was first described by Doege and Potter independently in 1930, which explains the eponym Doege-Potter syndrome (DPS)5. We report herein a rare case of DPS that showed malignant behavior.
A 60-year-old man was presented to us with loss of consciousness. He complained of disorientation and dyspnea on exertion. His initial blood sugar level was 37 mg/dL. He had no history of diabetes or medication, including of any hypoglycemic agent. He was diagnosed as hypertensive 10 years previously, and 5 years ago, was diagnosed with a spindle cell neoplasm after a biopsy taken from a 5├Ś6├Ś4-cm mass in his right hemithorax. However, he was not further evaluated and treated. His vital signs were as follows: body temperature, 37.1Ōäā; blood pressure, 150/90 mm Hg; pulse rate, 84 per minute; and respiratory rate, 20 per minute.
His initial physical examination revealed diminished breath sounds in his right middle and lower lung fields, and dullness to percussion. His cardiovascular examination was unremarkable.
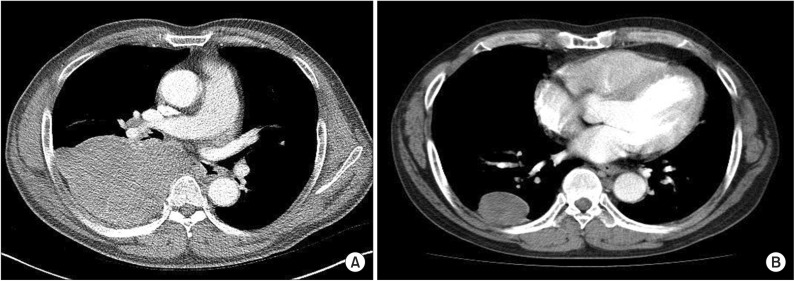
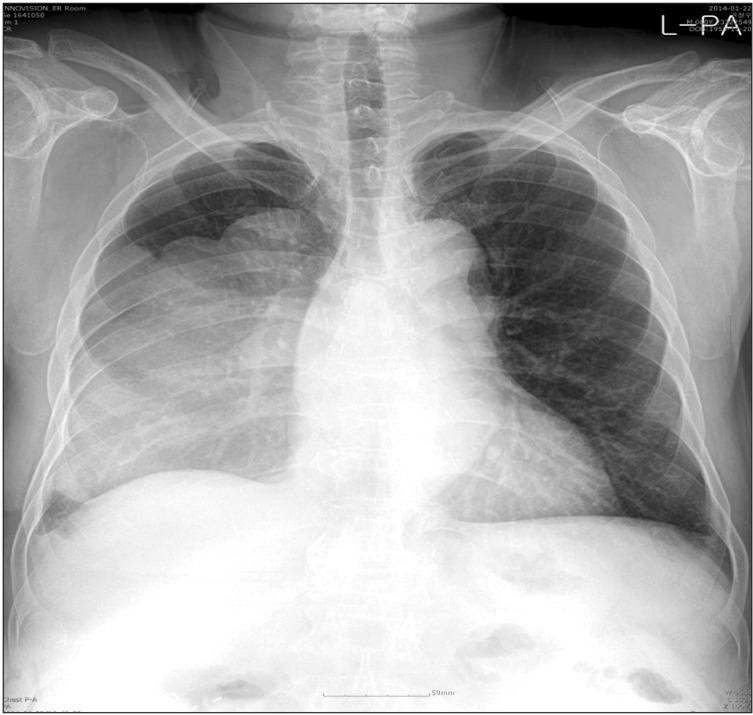
The patient's plain chest radiography showed large opacity in his right hemithorax (Figure 1). His contrast-enhanced chest computed tomography (CT) revealed a lobulated 15├Ś10├Ś19.5-cm-sized mass in his right hemithorax that showed heterogenous enhancement (Figure 2A). Compared with the chest CT images taken 5 years earlier (Figure 2B), the size of the mass increased from 5├Ś6├Ś4 cm to 15├Ś10├Ś19.5 cm. We could not perform a positron emission tomography scan because of severe hypoglycemia.
Endocrinologic laboratory test was performed to determine the cause of the hypoglycemia. The serum insulin and C-peptide level was suppressed (2.81 ┬ĄU/mL; normal range, 4-16 ┬ĄU/mL and 0.12 ng/mL; normal range, 1.07-3.51, respectively), which suggested that hypoglycemia did not resulted from either endogenous or exogenous hyperinsulinism. The blood cortisol, adrenocorticotrophic hormone, and growth hormone (GH) level was normal, and no insulin antibody was detected. We could not check the insulin-like growth factor II (IGF-II) level due to technical problem.
We performed a percutaneous needle biopsy of the mass. It revealed spindle cell neoplasm. Right thoracotomy revealed a large, multilobulated intrathoracic mass that caused atelectasis of the right lower lobe. We performed tumor resection and lobectomy of the right lower lobe. The tumor weighed 1,465.5 g and measured 18.5├Ś14├Ś11.5 cm.
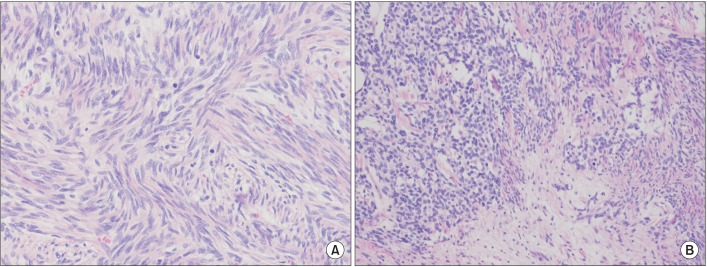
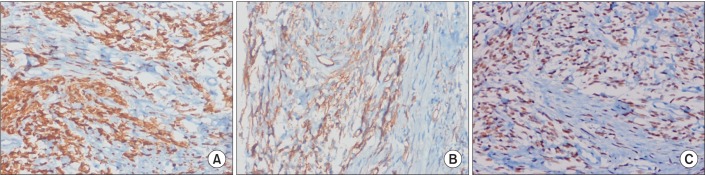
The histological finding was proliferation of spindle cells with a storiform-like and fascicular arrangement. The nuclear features showed a monotonous appearance. We also noted focal mild to moderate pleomorphism, and increased cellularity. The mitotic activity averaged 10-12 mitotic figures per 10 high-power fields (Figure 3). The tumor cells were immunohistochemically positive for CD34, Bcl-2, and STAT6, and negative for S-100 protein, CD117, actin, desmin, calretinin, D240, WT-1, and cytokeratin 5/6 (Figure 4). Based on the microscopic appearance of the immunohistological pattern, a final diagnosis of malignant SFTP was made.
After surgical resection of the tumor, the blood glucose levels became normal. The post-operative recovery was completed and the patient was discharged.
SFTP is a rare spindle cell neoplasm derived from mesenchymal cells1,6. It accounts for about 5% of all pleural neoplasms1,7. Since SFTP was first described in 1931 by Klemperer and Rabin8, about 800 cases of it have been reported in literature3. The frequency of SFTP is equal among patients of different ages, and there is no sex predilection9,10.
Macroscopically, tumors are usually lobular large masses that are well circumscribed with a smooth surface and often with a pedicle. Necrosis, hemorrhage, and calcification can be seen4.
Tumor diagnosis is based on histological analysis and an immunohistochemical study7. Microscopically, SFTP consists of ovoid or elongated spindle-shaped tumor cells with varying amounts of cytoplasms4,7. An immunohistochemical study is a useful tool for differentiating SFTP from different mesenchymal tumors, sarcomatous mesothelioma, and sarcoma3,4. Among the immunohistochemical markers, CD34 is very useful and characteristic, as it is often positive in most SFT cases and negative in most other pulmonary tumors11. STAT6 is very sensitive to SFPT, which is a specific immunohistochemical marker, and can be useful in CD34-negative SFTP12. Other markers that are positive in SFTP are vimentin, Bcl-2, and CD99, and negative markers in SFTP are S-100, carcinoembryonic antigen, and smooth muscle actin3,13. England et al.11 reported the pathological features of malignancy as high cellularity, marked cellular atypia, significant cellular polymorphism, prominent nucleoli, and high mitotic activity (greater than 4 mitoses per high-powered field)7,11. In our patient, the histological pattern revealed a spindle cell neoplasm. The positive CD34 and STAT6 findings were useful for the SFTP identification. The diagnosis of malignant SFTP was confirmed based on the criteria suggested by England et al.11
More than 50% of benign SFPT cases are asymptomatic and appear as an abnormality in plain chest radiography2,3. The symptoms are correlated with the tumor size and include cough, chest pain, and dyspnea4,11. Hemoptysis and obstructive pneumonitis rarely occur as results of airway obstruction3. Paraneoplastic syndromes may also occur and are associated with large tumors. Hypertrophic pulmonary osteoarthropathy (Pierre-Marie-Bamberg syndrome) has been described in 10%-20% of people with either benign or malignant SFTP3. Hypertrophic pulmonary osteoarthropathy (Pierre-Marie-Bamberg syndrome) has been described in 10%-20% of people with either benign or malignant SFTP3. Manifestations of hypoglycemia, known as DPS, very rarely occur, in less than 5% of cases7. Hypoglycemia is attributed to the production of the IGF-II, which lowers the blood glucose and impairs the GH's counter-regulatory response to hypoglycemia14. IGF-II may also stimulate glucose uptake by the tumor itself15. More cases of hypoglycemia occur with malignant SFTP with diameters of 10 cm or more11.
Endocrinologic test was perforemed to differentiate the causes of hypoglycemia. The low levels of serum insulin and C-peptide suggested not exogenous or endogenous hypoglycemia but IGF-mediated hypoglycemia. Nevertheless, IGF-II could not be checked in the hospital due to the technical limitations of this study. The initial treatment goal was normalization of the blood glucose level. To avoid hypoglycemia, the patient required a high-glucose diet and continuous intravenous infusion of 50% glucose (average of 15 g/hr). When we stopped the glucose infusion, the patient suffered from hypoglycemia. To lower the glucose infusion rate, we tried glucagon injection, but it had no effect. The overnight hyperglycemia was remarkable. Infusion of up to 50 g/hr of glucose was needed to prevent hypoglycemia. Considering the high possibility of the association of hypoglycemia with spindle cell neoplasm, surgical treatments were recommended for the patient. However, the patient strongly refused to undergo the surgery, which delayed the resection. In our case, the patient did not have hypoglycemia 5 years earlier, but symptomatic hypoglycemia occurred as the tumor growed, which suggest the association between the tumor size and the degree of hypoglycemia.
Therapies to reduce the tumor size include surgical resection, chemotherapy, radiotherapy, brachytherapy, and photodynamic therapy4. Complete surgical resection is a curative therapy for both benign and malignant SFPT4. Thoracotomy is required in the treatment of larger tumors, but small tumors can be resected through the video-assisted thoracoscopic approach4,10.
Despite the continuous intravenous glucose infusion, the patient agreed to undergo the surgery due to the blood vessel pain caused by the recurrent hypoglycemic episodes and the hypertonic glucose injection. After the tumor resection, the patient's blood glucose level normalized without glucose infusion.
The prognosis of benign SFTP with complete surgical resection is good10,11. In comparison, malignant SFTP has been reported to show a higher recurrence rate than benign SFTP. Most recurrences tend to occur within 24 months of the initial resection, but may happen even after more than 20 years4. Thus, malignant SFTP should be followed up with computer tomography of the chest to monitor its recurrence in two years3,4.
In conclusion, DPS is an uncommon paraneoplastic phenomenon with SFTP. In this case, SFTP was diagnosed based on the histological pattern and the immunohistochemical stain findings. No detailed association with IGF-II mediation was confirmed due to the lack of IGF-II measurements; but considering the large size of the malignant SFTP, the low insulin and c-peptide levels, and the normalized blood glucose level after the tumor resection, DPS was diagnosed.
Although DPS is very rare, its possibility must be considered in cases of refractory hypoglycemia. In addition, early diagnosis and surgical treatment must be performed in cases of a mass that had rapidly grown for five years.
References
1. Lee CE, Zanariah H, Masni M, Pau KK. Solitary fibrous tumour of the pleura presenting with refractory non-insulin mediated hypoglycaemia (the Doege-Potter syndrome). Med J Malaysia 2010;65:72-74. PMID: 21265256.

2. Campbell NA, Antippa PN. Solitary fibrous tumour of the pleura. Heart Lung Circ 2006;15:400-401. PMID: 16820321.


3. de Perrot M, Fischer S, Brundler MA, Sekine Y, Keshavjee S. Solitary fibrous tumors of the pleura. Ann Thorac Surg 2002;74:285-293. PMID: 12118790.


4. Robinson LA. Solitary fibrous tumor of the pleura. Cancer Control 2006;13:264-269. PMID: 17075563.


6. Leng XF, Xian L, Qin JJ, Lei BF. Malignant solitary fibrous tumor of pleura accompanied with first symptoms of chest pain and hemoptysis: a case report. Ann Thorac Cardiovasc Surg 2012;18:251-255. PMID: 22791000.


7. Briselli M, Mark EJ, Dickersin GR. Solitary fibrous tumors of the pleura: eight new cases and review of 360 cases in the literature. Cancer 1981;47:2678-2689. PMID: 7260861.


8. Klemperer P, Rabin CB. Primary neoplasm of the pleura: a report of five cases. Arch Pathol 1931;11:385-412.

9. Zhu Y, Du K, Ye X, Song D, Long D. Solitary fibrous tumors of pleura and lung: report of twelve cases. J Thorac Dis 2013;5:310-313. PMID: 23825765.


10. Magdeleinat P, Alifano M, Petino A, Le Rochais JP, Dulmet E, Galateau F, et al. Solitary fibrous tumors of the pleura: clinical characteristics, surgical treatment and outcome. Eur J Cardiothorac Surg 2002;21:1087-1093. PMID: 12048090.



11. England DM, Hochholzer L, McCarthy MJ. Localized benign and malignant fibrous tumors of the pleura: a clinicopathologic review of 223 cases. Am J Surg Pathol 1989;13:640-658. PMID: 2665534.


12. Doyle LA, Vivero M, Fletcher CD, Mertens F, Hornick JL. Nuclear expression of STAT6 distinguishes solitary fibrous tumor from histologic mimics. Mod Pathol 2014;27:390-395. PMID: 24030747.



13. Chilosi M, Facchettti F, Dei Tos AP, Lestani M, Morassi ML, Martignoni G, et al. bcl-2 expression in pleural and extrapleural solitary fibrous tumours. J Pathol 1997;181:362-367. PMID: 9196431.


14. Masson EA, MacFarlane IA, Graham D, Foy P. Spontaneous hypoglycaemia due to a pleural fibroma: role of insulin like growth factors. Thorax 1991;46:930-931. PMID: 1792643.



15. Schutt RC, Gordon TA, Bhabhra R, Cathro HP, Cook SL, Mc-Cartney CR, et al. Doege-Potter syndrome presenting with hypoinsulinemic hypoglycemia in a patient with a malignant extrapleural solitary fibrous tumor: a case report. J Med Case Rep 2013;7:11PMID: 23302323.




Figure┬Ā2
Contrast-enhanced chest computed tomography (CT) revealed a lobulated 15├Ś10├Ś19.5-cm-sized mass in his right hemithorax that showed heterogenous enhancement (A). Compared with the chest CT images taken 5 years earlier (B), the size of the mass had increased.
Figure┬Ā3
Histopathological findings. (A) The tumor showed a short intersecting fascicular pattern, composed of spindle cells with mild atypia, hypercellularity, and high mitotic figures (average 10-12/10 HPF) (H&E stain, ├Ś200). (B) There is coexistence of hypo- and hypercellular areas. The spindle cells show moderate atypism with ropy collagenous stroma. Mitotic figures were less than 4/10 HPF (H&E stain, ├Ś200).
- TOOLS
-
METRICS

- Related articles
-
A Case of Benign Mesothelioma Associated with Hypoglycemia1990 December;37(4)
A Case of Malignant Mesothelioma of Pleura Associated with Hypoglycemia1990 December;37(4)
A Case Report of Polyarteritis Nodosa Associated with Alveolar Hemorrhage1990 March;37(1)
A Case of Nodular Lesions in Lung Associated with Dyspnea1989 June;36(2)
A Case of Diffuse Alveolar Hemorrhage Associated with Overlap syndrome1988 September;35(3)


 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Print
Print Download Citation
Download Citation



