Does the Mean Arterial Pressure Influence Mortality Rate in Patients with Acute Hypoxemic Respiratory Failure under Mechanical Ventilation?
Article information
Abstract
Background
In sepsis patients, target mean arterial pressures (MAPs) greater than 65 mm Hg are recommended. However, there is no such recommendation for patients receiving mechanical ventilation. We aimed to evaluate the influence of MAP over the first 24 hours after intensive care unit (ICU) admission on the mortality rate at 60 days post-admission in patients showing acute hypoxemic respiratory failure under mechanical ventilation.
Methods
This prospective, multicenter study included 22 ICUs and compared the mortality and clinical outcomes in patients showing acute hypoxemic respiratory failure with high (75-90 mm Hg) and low (65-74.9 mm Hg) MAPs over the first 24 hours of admission to the ICU.
Results
Of the 844 patients with acute hypoxemic respiratory failure, 338 had a sustained MAP of 65-90 mm Hg over the first 24 hours of admission to the ICU. At 60 days, the mortality rates in the low (26.2%) and high (24.5%) MAP groups were not significantly different. The ICU days, hospital days, and 60-day mortality rate did not differ between the groups.
Conclusion
In the first 24 hours of ICU admission, MAP range between 65 and 90 mm Hg in patients with acute hypoxemic respiratory failure under mechanical ventilation may not cause significantly differences in 60-day mortality.
Introduction
Acute hypoxemic respiratory failure (AHRF) is a common disease in the intensive care unit (ICU), and frequently requires mechanical ventilation. The condition may be caused by left ventricular failure, increased alveolar capillary permeability as observed in acute respiratory distress syndrome (ARDS), diffuse alveolar haemorrhage, or inflammatory exudates such as those observed in pneumonia or other inflammatory lung conditions1. AHRF in mechanically ventilated patients can present in an ARDS or non-ARDS form. Acute respiratory failure occurs in 56% of all patients in the ICU and the mortality rate is 34%2. In other cohort studies, the mortality rates in AHRF patients were found to be higher than 40%3,4.
The role of arterial blood pressure on outcomes and mortality is well studied in shock patients5,6. However, there is paucity of data about the role of mean arterial pressure (MAP) in AHRF patients under mechanical ventilation. One study found that impaired perfusion of small vessels caused alterations in microcirculation and had an important effect on outcomes and survival in patients with septic shock7. Additional studies conducted on patients with septic shock found that increases in MAP from 65 mm Hg to 85 mm Hg were not related to tissue perfusion or mortality8,9. However, both the effect of MAP on mortality rate and the maintenance of a blood pressure target in shock patients remain controversial.
Mechanical ventilation is critical for the survival of most ICU patients with AHRF. However, over-inflation of the lung, which frequently occurs with mechanical ventilation, raises the stresses in the capillary walls to high levels. This leads to ultrastructural changes in the barrier, a condition known as stress failure10. As a result, mechanical ventilation itself may cause additional lung injury, affecting survival in ICU patients11,12,13.
The Surviving Sepsis Campaign has suggested maintaining a target MAP higher than 65 mm Hg in cases of septic shock14. However, there is no such recommendation for mechanically ventilated patients. We hypothesized that MAP may influence survival in mechanically ventilated patients with AHRF. We divided mechanically ventilated patients with AHRF into two groups depending on their MAP over the first 24 hours after ICU admission: the first low MAP group consisted of individuals with MAP ranging from 65 to 74.9 mm Hg and the second high MAP group consisted of individuals with MAP from 75 to 90 mm Hg.
Materials and Methods
1. Subjects and study design
This study is a subgroup analysis of a prospective cohort study that consisted of patients from 22 ICUs in Korea, and data from the "Validation of Simplified Acute Physiology Score 3 in Korean ICUs (VSKI)" study. Participating hospital units were enrolled voluntarily. The VSKI study included 22 ICUs (14 medical, 6 surgical, and 2 multidisciplinary) in 15 tertiary-level hospitals. Approval from the respective ethics committees was obtained at each hospital. All adult patients with AHRF admitted to the participating ICUs were screened regardless of ARDS status starting on July 1, 2010, through to January 31, 2011. The patients with a PaO2/FIO2 ratio less than 300 mm Hg at ICU admission during the study period formed the study cohort. The patients were classified into two groups according to MAP over the first 24 hours: the low MAP group (65-74.9 mm Hg) and the high MAP group (75-90 mm Hg).
2. Data collection
Demographic information including age, gender, MAP, body mass index, co-morbid conditions, acute lung injury (ALI) risk factors, vital signs, and laboratory features were recorded. The disease severity was assessed based on the Simplified Acute Physiology Score III (SAPS III)15 and the Sequential Organ Failure Assessment (SOFA) score16 at ICU admission. The duration of hospitalization and duration in the ICU was also measured for each patient. Patients were followed up for 60 days following hospital admission.
3. Outcomes
The primary outcome of this study was the 60-day mortality rate for AHRF with high (75-90 mm Hg) and low (65-74.9 mm Hg) MAP over the first 24 hours of ICU admission. Secondary outcomes included the durations of hospital and ICU stay in both groups.
4. Statistical analysis
Data were presented as mean (standard deviation) for continuous variables and the number of subjects (%) for categorical variables. Categorical variables were compared using the Fisher exact test or Pearson χ2 test as appropriate, and continuous variables were compared by the Mann-Whitney U test or Student t test, after determining normality using the Kolmogorov-Smirnov test. All tests for significance were two-tailed, and a p-value of less than 0.5 was considered significant. Multivariate linear (for continuous variables) and logistic (for categorical variables) regressions were used to evaluate the association between MAP in the first 24 hours of admission and survival. Survival curves for both study groups were compared using the log-rank test. Results were analyzed with SPSS version 20.0 for Windows (SPSS Inc., Chicago, IL, USA).
Results
Of the 844 patients diagnosed with AHRF, 338 individuals fulfilled the required criteria for our study (Figure 1). The population demographics for patients with low MAP over the first 24 hours as compared to the patients with high MAP over the first 24 hours is summarized in Table 1. The mean patient age in both groups was 60 years, and 65% of all patients were men. Sepsis was the most probable cause of AHRF in 26% of patients, while cancer and diabetes were the most common co-morbidities, affecting 89 (26%) and 79 (23%) patients, respectively. Chronic obstructive pulmonary disease as a co-morbidity occurred in a significantly higher number of patients with high MAP than in patients with low MAP. Meanwhile sepsis affected higher percentage of patients with low MAP than those with high MAP. The high MAP group had a significantly higher PaO2/FIO2 ratio and C-reactive protein than the low MAP group. ARDS was present in 20% of the patients with low MAP, and in 22.4% of the patients with high MAP. The duration of ICU stay, hospital stay, and 60-day mortality rate did not differ between the two groups.

Flow chart of patients who sustained mean arterial pressure (MAP) of 65 to 90 mm Hg over the first 24 hours of the intensive care unit (ICU) admission among hypoxemic respiratory failure.

Patient demographics, risk factors, co-morbidities, and clinical outcomes of 338 AHRF patients admitted to the ICU
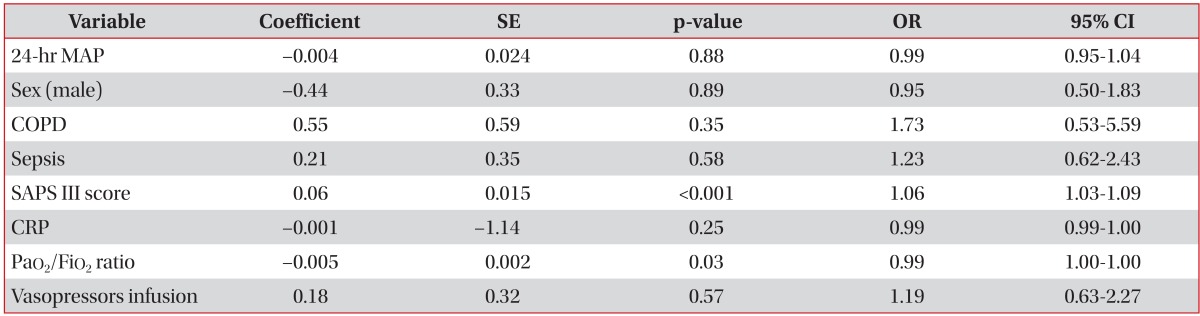
A multivariate analysis was performed to evaluate the independent factors affecting the 60-day mortality rate in mechanically ventilated AHRF patients. The MAP in the first 24 hours is not an independent factor associated with 60-day mortality rate. We found that the SAPS III score and the PaO2/FiO2 ratio are independently associated with 60-day mortality rate (Table 2).
The Kaplan-Meier survival curves demonstrated the same survival rates for patients with high and low MAP over the first 24 hours of admission to the ICU. The 60-day mortality rate was approximately 60% for both study groups (Figure 2).

Kaplan-Meier survival curves: patients with low mean arterial pressure (MAP) (65-74.9 mm Hg) (solid line) compared to patients with high MAP (75-89.9 mm Hg) (dotted line).
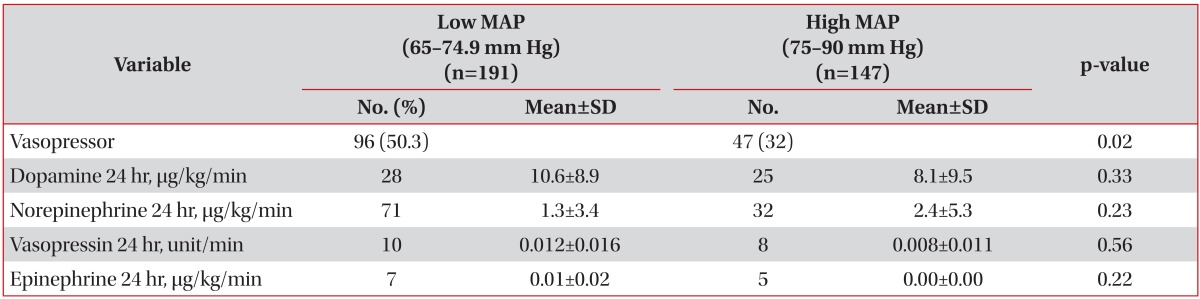
To ensure that the MAP was maintained in excess of 65 mm Hg in our study patients, we used fluid perfusions and vasopressors. The number of patients receiving vasopressors in the low MAP group was significantly higher than in the high MAP group. The mean dosage for each vasopressor used did not differ between the two groups. Norepinephrine alone or combined with dopamine was used in most patients (Table 3).
Discussion
To clarify the relationship between MAP observed over the first 24 hours of admission and the 60-day mortality rate, and ICU outcomes in patients with AHRF, we performed a subgroup analysis of a prospective, multicenter study in 22 ICUs. We divided the patients that fulfilled our study criteria into two groups according to MAP over the first 24-hour post-admission; a low MAP group (65-74.9 mm Hg), and high MAP group (75-90 mm Hg). We found that there was no difference in 60-day mortality rates between the patients with high and low MAP.
Several other studies have used a range of 65-85 mm Hg as an MAP target for the resuscitation of septic shock patients8,9. In our study, the MAP over the first 24 hours was 69.6 mm Hg and 80.4 mm Hg for the low and high MAP groups, respectively. Additionally, in several clinical trials that enrolled patients with ALI or ARDS under mechanical ventilation, the baseline MAP at the time of admission was approximately 76-77 mm Hg. They aimed to maintain admission MAP over the first 24 hours post-admission17,18. However, the optimal arterial blood pressure level in mechanically ventilated AHRF patients is not well known. Therefore, we used an MAP of 65-74.9 mm Hg as a target range, as recommended by the surviving sepsis campaign19 and 75-90 mm Hg as a high range of MAP.
In a study conducted by Asfar et al.20, they showed that the mortality rate of septic shock patients was not related with the MAP. They found the 28-day mortality rate for the high target group (MAP, 80-85 mm Hg) to be 36.6%, and 34.0% for low target group (MAP, 65-70 mm Hg)20. Our study showed that the mortality rates of mechanically ventilated patients did not change in relation to MAP, when maintained above 65 mm Hg. We found 60-day mortality rates in patients with an MAP of 65-74.9 mm Hg to be 26.2%, and 24.5% in patients with an MAP of 75-90 mm Hg, with no statistically significant difference between them.
In the present study, the percentage of patients under vasopressor therapy was lower (42%) than previous randomized trials on ARDS patents (73%)21. Furthermore, in the high MAP group in our study, less than 20% of patients received vasopressor therapy. Multivariate analysis showed vasopressor therapy was not related with 60-day mortality in this study. This finding may help to target blood pressure in mechanically ventilated patients, other than those with underlying sepsis. We used norepinephrine in 37% of the patients with MAP of 65-74.9 mm Hg and in 21% of the patients with MAP of 75-90 mm Hg. Norepinephrine was used alone or combined with dopamine. Epinephrine and vasopressin were used in fewer patients. The literature reports mean values of norepinephrine perfusion on the first day of admission for septic shock patients ranging from 0.26-0.94 µg/kg/min in different studies20,22,23,24,25,26. In our study, the mean norepinephrine perfusion rate ranged from 1.3-2.4 µg/kg/min, which is higher than previous studies. The highest dose of norepinephrine reported was 3.3 µg/kg/min27.
We administered almost the same quantity of fluids in both groups of patient. The fluid perfusion dosage in shock patients is previously described as ranging from 1,500-3,500 mL20,22,23,24,25,26. The urine output between patients with MAP of 65-74.5 mm Hg and 75-90 mm Hg did not differ significantly in the present study. This is in a good agreement with the results of other studies8,9. In 2000, LeDoux et al.8 found no difference in systemic oxygen metabolism, skin microcirculatory blood flow, urine output, or splanchnic perfusion between patients with MAP ranging between 65-85 mm Hg. In 2009, in a study conducted by Dubin et al.28, the increase in arterial blood pressure with norepinephrine did not improve the microcirculatory blood flow.
Despite the fact that sepsis is the most common cause of AHRF, accounting for approximately 26% of cases and occurring most frequently in the low MAP group, the percentage of sepsis in our study is lower than that observed in other ALI/ARDS clinical trials21. In a French randomized controlled trial, approximately 61% of the patients had sepsis as a co-morbidity29. However, in another clinical trial, only 22% of the patients were diagnosed with sepsis18.
A multivariate analysis showed that SAPS III score and the PaO2/FiO2 ratio were significantly associated with 60-day mortality rates in patients with AHRF under mechanical ventilation. The duration of the ICU stay, hospital stay, and 60-day mortality rate did not differ between the two groups.
In our study, the 60-day mortality rate was 26.2% and 24.5% for patients with MAP of 65-74.9 mm Hg and 75-90 mm Hg, respectively. The SAPS III score in the low group was 67.8 and 61.6 in the high group. According to The National Heart, Lung and Blood Institute ARDS Network trials, the 60-day mortality rate has decreased dramatically over the past two decades. In 2004-2005, it was reported to be 26%30, and in a more recent ARDS Network clinical trial, a 60-day mortality rate of 22% was reported31,32,33.
In conclusion, 60-day mortality is not affected by first 24 hours MAP of 65-74.9 mm Hg, as compared with 75 to 90 mm Hg for patients with AHRF under mechanical ventilation.
Acknowledgements
We thanks to all of the investigators conducting "Validation of Simplified Acute Physiology Score 3 in Korean ICUs" (VSKI) study.
Notes
Conflicts of Interest: No potential conflict of interest relevant to this article was reported.