An Increased Proportion of Apoptosis in CD4+ T Lymphocytes Isolated from the Peripheral Blood in Patients with Stable Chronic Obstructive Pulmonary Disease
Article information
Abstract
Background
The pathophysiology of chronic obstructive pulmonary disease (COPD) includes inflammation, oxidative stress, an imbalance of proteases and antiproteases and apoptosis which has been focused on lately. Abnormal apoptotic events have been demonstrated in both epithelial and endothelial cells, as well as in inflammatory cells including neutrophils and lymphocytes in the lungs of COPD patients. An increased propensity of activated T lymphocytes to undergo apoptosis has been observed in the peripheral blood of COPD patients. Therefore, the apoptosis of T lymphocytes without activating them was investigated in this study.
Methods
Twelve control subjects, 21 stable COPD patients and 15 exacerbated COPD patients were recruited in the study. The T lymphocytes were isolated from the peripheral blood using magnetically activated cell sorting. Apoptosis of the T lymphocytes was assessed with flow cytometry using Annexin V and 7-aminoactinomycin D. Apoptosis of T lymphocytes at 24 hours after the cell culture was measured so that the T lymphocyte apoptosis among the control and the COPD patients could be compared.
Results
Stable COPD patients had increased rates of CD4+ T lymphocyte apoptosis at 24 hours after the cell culture, more than the CD4+ T lymphocyte apoptosis which appeared in the control group, while the COPD patients with acute exacerbation had an amplified response of CD4+ T lymphocyte apoptosis as well as of CD8+ T lymphocyte apoptosis at 24 hours after the cell culture.
Conclusion
Stable COPD patients have more apoptosis of CD4+ T lymphocytes, which can be associated with the pathophysiology of COPD in stable conditions.
Introduction
Chronic obstructive pulmonary disease (COPD) can be caused by interactions between environmental factors such as smoking, occupational exposure, indoor pollution, and host factors including genetic predisposition1.
When noxious particles such as smoking or biomass come into the airways in susceptible individuals, various inflammatory cells including macrophages, neutrophils, and lymphocytes are recruited to induce the cascade of chronic inflammation2. Imbalance between proteases and antiproteases pivoting toward proteolytic activity results in parenchymal destruction of the lungs3. In addition, oxidative stress is associated with neutrophil infiltration, mucus secretion, inactivation of antiproteases, and apoptosis4. Those pathways of pathophysiology in COPD interact one another rather than exerting their effects respectively. Lately apoptosis of inflammatory cells as well as structural cells in the lungs has been forwarded among the pathophysiology.
Apoptosis is a strictly regulated mechanism in cell metabolism, eliminating non-viable cells which has been injured or infected. It helps maintain cellular homeostasis balancing cellular proliferation and cellular removal. Lung tissues from COPD patients showed increased apoptosis in epithelial, endothelial, and interstitial cells567. In a controlled study, emphysema group showed more cellular apoptosis than cellular proliferation8. Apoptotic cells are rarely found in the human lungs of normal condition, suggesting that either rapid removal process or low rates of cell death9. Apoptosis in COPD can be part of the pathophysiology involving the following mechanisms10. First, inflammatory cells such as macrophages, neutrophils, and CD8+ T lymphocytes are recruited in the lungs of COPD patients11. Such cytotoxic CD8+ T lymphocytes seem to be associated with apoptosis of alveolar epithelia through mediation of perforin, granzyme-B, and tumor necrosis factor α12. Second, inadequate cell-matrix interactions lead to apoptosis of the cells through the decrease in survival signals13. Third, oxidative stress can reduce vascular epithelial growth factors by epithelial injury, causing apoptosis of alveolar cells prompting progression of emphysematous changes of the lungs14.
The enhanced apoptosis of T lymphocytes in airways of COPD patients has been reported15. This enhanced apoptosis of airway T lymphocytes could potentially result in secondary necrosis, retention of apoptotic material, and perpetuation of the inflammation in COPD16. Interestingly, T lymphocytes isolated from peripheral blood in COPD patients had an increased propensity for apoptosis16. The levels of apoptosis in phytohemagglutinin (PHA)-stimulated T lymphocytes isolated from peripheral blood showed increased apoptosis of T lymphocytes without significant difference of apoptosis between CD4+ and CD8+ T lymphocytes16. Therefore, this research was aimed to study the different phenotype of apoptotic T lymphocytes in stable COPD without stimulating them with PHA. If the relative increase in CD8+ T lymphocytes in peripheral blood in COPD patients17 is associated with the different peripheral blood T lymphocyte apoptosis between CD4+ and CD8+ T cells, we can understand more about the pathophysiology of COPD. Since lymphocytes in the bronchoalveolar space are known to traffic from the bloodstream and subsequently rejoin the peripheral circulation18, the relative increase in peripheral blood cytotoxic T lymphocytes resulting from the different apoptosis between CD4+ and CD8+ cells could explain the pathophysiology of COPD17. Furthermore, it could be associated with the apoptotic events involving inflammatory cells and structural cells of the lungs in COPD patients12171920.
Materials and Methods
1. Study subjects
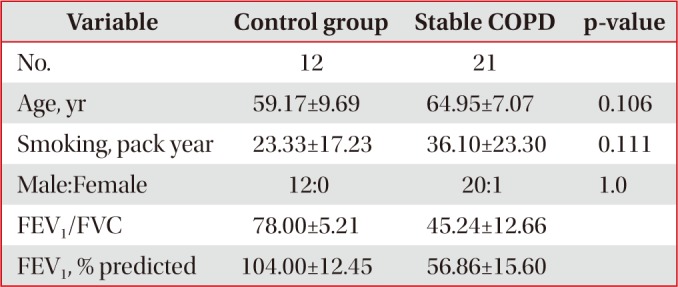
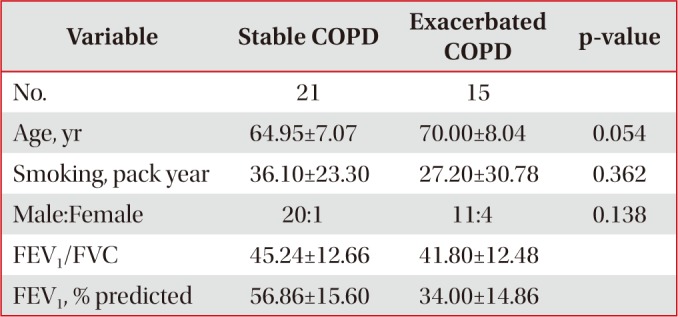
Twelve control subjects, 21 stable COPD patients, and 15 exacerbated COPD patients with smoking histories including current and ex-smokers were recruited in the study after obtaining informed consent. They were excluded, when they had malignancies, active infectious diseases, or severe medical diseases such as liver or renal problems affecting their respiratory symptoms. Control group was defined as healthy smokers without documented pulmonary diseases including bronchial asthma or COPD, when they showed more than 70% of the ratio of post-bronchodilator forced expiratory volume in 1 second (FEV1)//forced vital capacity (FVC), and more than 80% of the predicted value of post-bronchodilator FEV1. Stable COPD group was included when their spirometry results showed less than 70% of the ratio of post-bronchodilator FEV1/FVC, and less than 80% of the predicted value of post-bronchodilator FEV11. Exacerbated COPD group was included when COPD patients were admitted to the hospital due to the aggravation of their respiratory symptoms such as dyspnea, increase in sputum amount or sputum purulency. All measurements were taken in exacerbated COPD patients within 24 hours after hospital admission.
2. Collecting peripheral blood with isolation of T lymphocytes
Ten milliliters of peripheral blood was drawn into vacutainer (Becton Dickinson, Oxford, UK) from control group and COPD patients, and the samples were placed to 15 mL of polypropylene tube containing 4 mL of lymphoprep (Axis-Shield PoC AS, Oslo, Norway) to centrifuge 2,000 rpm for 20 minutes with separation of monocytes and lymphocytes from the blood. The separated cells were washed by phosphate buffered saline (Gibco, Grand Island, NY, USA) two times. Forty microliters of MACS buffer consisting of phosphate buffered saline, 0.5% bovine serum albumin, and 2 mL ethylenediaminetetraacetic acid with 10 µL of anti-CD3 magnetic beads (Miltenyi Biotech, Bergisch Gladbach, Germany) per 107 of cells were added to the samples. T lymphocytes were isolated by magnetically activated cell sorting (MACS; Miltenyi Biotech) putting them through MACS column following washing them with 10 mL of MACS buffer after 15 minutes of dark incubation at 4℃. The samples were cultured for 24 hours in RPMI 1640 culture medium (Gibco) containing 10% FBS and 1% penicillin-streptomycin (Gibco).
3. Measuring apoptosis
Ten microliters of CD4 APC antibody, and 10 µL of CD8 APC antibody (Becton Dickinson) per 107 of cells were added to the samples to differentiate between CD4+ T lymphocytes and CD8+ T lymphocytes. Five microliters of 7-aminoactinomycin D (7AAD)-FITC (Becton Dickinson) and 5 µL of Annexin V-FITC (Becton Dickinson) were added to the T lymphocytes after 20 minutes of dark incubation at 4℃, and the samples were incubated for 15 minutes to measure apoptosis of the cells with FACScalibur (Becton Dickinson). Annexin V-positive/7AAD-negative cells were defined as early apoptosis, while Annexin V-positive/7AAD-positive cells were defined as late apoptosis21. Therefore, apoptosis of T lymphocytes was defined as the sum of early apoptosis and late apoptosis (Figure 1).
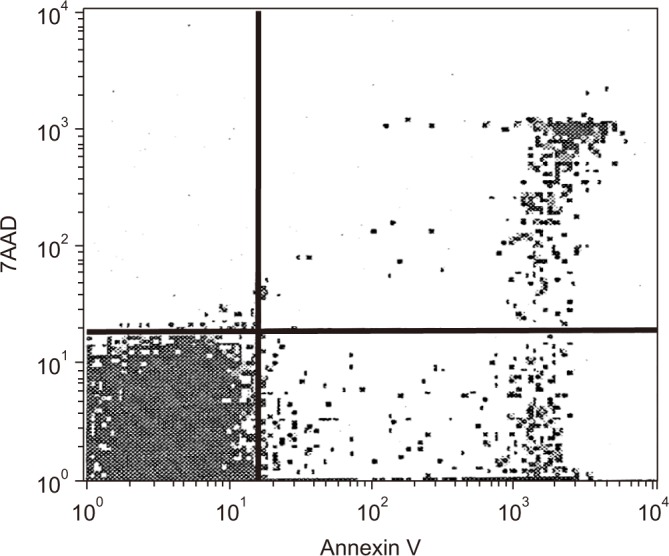
Annexin V vs. 7-aminoactinomycin D (7AAD) staining. The percentage of Annexin V-positive/7AAD-negative cells (representing cells in the early stage of apoptosis) and Annexin V-positive/7AAD-positive cells (representing cells in the late stage of apoptosis) were measured using flow cytometry with a FACScalibur system.
4. Statistical analysis
Continuous data was expressed by mean and standard deviation and categorical data was expressed by frequency. The difference of continuous variables between groups was compared with unpaired T-student test. Discrete variables were compared using Fisher exact test. The correlation between variables was checked with Pearson's correlation analysis. When p-value was less than 0.05, it was considered significant.
Results
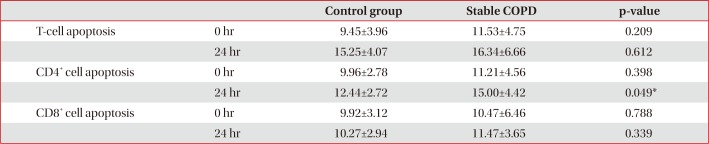
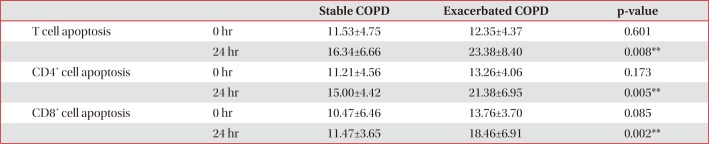
Control and stable COPD groups showed no demographic difference in age, sex, and smoking (Table 1). Our data was age and smoking status matched between control group and stable COPD, which was new to the previous study designs. Stable COPD patients had increased rates of CD4+ T lymphocyte apoptosis at 24 hours after cell culture more than CD4+ T lymphocyte apoptosis in control group (12.44±2.72% of CD4+ T lymphocyte apoptosis in control group vs. 15.00±4.42% of CD4+ T lymphocyte apoptosis in stable COPD, p=0.049) (Table 2). In addition, apoptosis of CD4+ T lymphocytes correlated with apoptosis of CD8+ T lymphocytes at 0 hour and at 24 hours after cell culture in stable COPD (Figure 2). Stable and exacerbated COPD groups showed no demographic difference in age, gender, and smoking (Table 3). COPD patients with acute exacerbation had amplified response of CD4+ T lymphocyte apoptosis as well as of CD8+ T lymphocyte apoptosis at 24 hours after cell culture (Table 4).
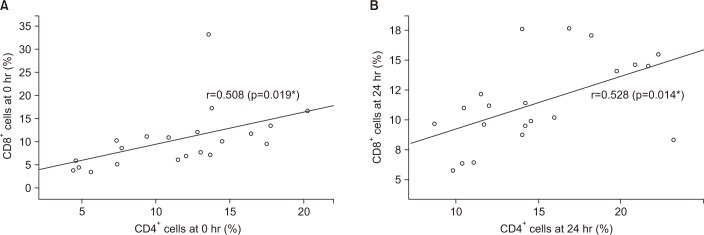
(A) Correlation of apoptosis between CD4+ T lymphocyte and CD8+ T lymphocyte in patients with stable chronic obstructive pulmonary disease (COPD) at 0 hour. (B) Correlation of apoptosis between CD4+ T lymphocyte and CD8+ T lymphocyte in patients with stable COPD at 24 hours after culture. r=Pearson correlation coefficient, *p<0.05.
Discussion
The main finding of this study includes the increased proportion of CD4+ T lymphocyte apoptosis in stable COPD than control group, which was a new finding different from the previous reports16. Since T lymphocytes collected from peripheral blood in COPD patients showed an increased propensity for apoptosis16, the differentiation of apoptosis between CD4+ T lymphocytes and CD8+ T lymphocytes could answer the question about the relative increase in CD8+ T lymphocytes in COPD17, and the difference of T lymphocyte apoptosis between non-COPD population and COPD population.
The purpose of the study was to find the different phenotype of T lymphocyte apoptosis between control group and stable COPD, because the phenotype of PHA-stimulated T lymphocytes collected from peripheral blood had no significant difference of apoptosis between CD4+ T lymphocytes and CD8+ T lymphocytes16. In this study, apoptosis of T lymphocytes was studied using MACS for isolation of T lymphocytes without activation. The difference in apoptosis between CD4+ T lymphocytes and CD8+ T lymphocytes in stable COPD patients showing the increased CD4+ T lymphocyte apoptosis in COPD was demonstrated in this study (Table 2). Considering the increased proportion of CD4+ T lymphocyte apoptosis in stable COPD without the difference of total T lymphocyte apoptosis from control group, we can infer that such T lymphocyte apoptosis would lead to the relative increase in CD8+ T lymphocytes in stable COPD. Given the results of the increased CD4+ T lymphocyte apoptosis in COPD, we can understand the reason why CD8+ T lymphocytes, which are in the center of pathophysiology of COPD, are outnumbered in COPD by CD4+ T lymphocytes. Even though the environment of cell culture was not in vivo, but the status without cell activation could be another model of T lymphocyte apoptosis.
When we speculate about the different results of T lymphocyte apoptosis in this study from the previous ones, the difference might have originated from the following reasons. First, this study did not stimulate T lymphocytes with PHA. Under normal circumstances, apoptosis is followed by rapid phagocytosis of apoptotic cells by monocytes or macrophages. In the same way, the detectable level of apoptotic T cells in peripheral blood without stimulation is quite low, which is likely a reflection of the rapid removal of apoptotic cells from the circulation22. The comparisons of apoptotic T cells at 0 hour after collection from the peripheral blood between groups are thus prone to miss significant differences between groups. To investigate the propensity of T lymphocyte apoptosis, previous studies used PHA for stimulation16. When we use MACS, we can also enrich the detectable level of apoptotic T lymphocytes23. The cell cultures would be able to demonstrate the different propensity of apoptosis in the T lymphocytes rather than quantitative comparison of apoptosis24. In this study, the significant difference of T lymphocyte apoptosis between groups was found after cell culture using MACS (Tables 2, 4). The results in this study also showed that apoptosis of CD4+ T lymphocytes correlated with apoptosis of CD8+ T lymphocytes (Figure 2), suggesting that the previous results of apoptosis of T lymphocytes without significant difference between CD4+ T lymphocytes and CD8+ T lymphocytes could be associated with T lymphocyte activation with PHA before measuring the degree of apoptosis16. The author assumed that activation of T lymphocyte with PHA could amplify the apoptotic response of T lymphocytes leading to apoptosis of the CD4+ and CD8+ T lymphocytes. In addition, the comparative results of T lymphocyte apoptosis between stable and exacerbated COPD in this study supported the hypothesis. The activated T lymphocytes in exacerbated COPD showed increased apoptosis of CD4+ and CD8+ T lymphocytes in this study (Table 4). The results imply that T lymphocyte activation can lead to the increase in apoptosis of both CD4+ and CD8+ T lymphocytes. Such enhanced apoptosis of activated T lymphocytes would amplify the inflammatory response of COPD. Consequently, acute exacerbations in COPD patients can contribute to progressive tissue damage and bronchial obstruction25. Second, these results of the study are age and smoking history matched between the groups. The previous studies were not age or smoking status matched, which could account for the different results16. Third, apoptosis studies on T lymphocytes used different modalities comprising PHA stimulation, or positive selection of multiple T-cell markers using MACS that could potentially activate T lymphocytes26. Therefore, comparative studies about the modalities of cell separation regarding apoptosis would be required to elucidate the difference.
The aim of the study was to find the different phenotype of apoptotic T lymphocytes between control group and stable COPD. We have the following areas to consider interpreting the results of this study. First, the number of enrolled groups was small, preventing subgroup analysis of different clinical phenotypes of COPD. Second, we found the different apoptosis of T lymphocytes between control group and stable COPD, but we did not quantify the T lymphocytes according to T cell markers due to our technical limitations. We suggest that more advanced technology would enable researchers to perform quantitative analysis of T lymphocyte apoptosis. Third, there is a possibility that comorbidities could have influenced the results of T lymphocyte apoptosis, which warrants studies investigating the relationship between apoptosis of T lymphocytes and systemic inflammation in COPD.
In conclusion, this study suggested that the increased proportion of CD4+ T lymphocyte apoptosis could be the reason of the relative increase in CD8+ T lymphocytes in COPD14. In addition, apoptosis of CD4+ T lymphocytes in stable COPD patients can be associated with pathophysiology of COPD in stable conditions.
Acknowledgments
The author thanks Mi-Ran Kim for her excellent assistance in the experiment, and Yun-Ju Kim for her excellent assistance in recruiting control group and stable COPD.
Notes
Conflicts of Interest: No potential conflict of interest relevant to this article was reported.