 |
 |
| Tuberc Respir Dis > Volume 77(1); 2014 > Article |
|
Abstract
The recovery of nontuberculous mycobacteria (NTM) from respiratory specimens and the number of patients with NTM lung disease have been rapidly increasing in Korea. An early differential diagnosis of NTM lung disease from pulmonary tuberculosis (TB) is important, as the therapeutic regimen differs from that of pulmonary TB, and it is not necessary to track the contacts of patients with NTM lung disease. However, differentiating NTM lung disease from pulmonary TB remains difficult, because the clinical presentations of the two diseases are similar and a definite diagnosis of NTM lung disease based on sputum culture takes time. This review focuses on the changing epidemiology, clinical and radiographic manifestation, and laboratory diagnosis of pulmonary TB and NTM lung disease in Korea.
Nontuberculous mycobacteria (NTM) refer generally to mycobacteria other than Mycobacterium tuberculosis complex (MTB) and Mycobacterium leprae. The prevalence of lung diseases caused by NTM is increasing worldwide, affecting both immunocompetent and immunocompromised individuals1,2. In addition, the distribution of NTM variants is not uniform, and there is marked geographic variability in the prevalence of NTM lung disease and the mycobacterial species responsible3,4.
In Korea, tuberculosis (TB) remains a serious public health problem. In countries with a high TB prevalence, patients whose sputum tests positive for acid-fast bacilli (AFB) on direct microscopic examination or those displaying chest radiographic findings that suggest active TB are generally presumed to have pulmonary TB and are treated empirically with anti-TB drugs. This results in incorrect diagnoses and has led to inappropriate or unnecessary treatment of many patients with NTM lung disease5,6. An early differential diagnosis of NTM lung disease from pulmonary TB is important, as the therapeutic regimen differs from that of pulmonary TB and it is not necessary to track the contacts of patients with NTM lung disease7. However, differentiating NTM lung disease from pulmonary TB remains difficult because clinical presentations of the two diseases are similar and a definite diagnosis of NTM lung disease based on sputum culture takes time7,8.
When NTM are cultured and identified in respiratory specimens, a diagnosis of NTM lung disease requires differentiation from contamination or colonization1,2. Thus, the detection of AFB in respiratory specimens, or the isolation of NTM species, may pose a diagnostic problem for the clinician1,2. In recent years, this issue has become even more relevant, as the frequency of NTM detection in respiratory specimens, as well as the number of patients with NTM lung disease, continues to increase rapidly in Korea.
In this article, we review the changing epidemiology, clinical and radiographic manifestations, and laboratory diagnoses of pulmonary TB and NTM lung disease in Korea.
In Korea, the prevalence of active TB has decreased from 5,065 in 1965 to 1,032/100,000 population in 19959. However, TB is still a major health problem and reported TB cases have not significantly decreased over the last decade10, being 100.6 in 2001 and 87.6/100,000 population in 201211. Unlike TB, NTM lung disease does not have to be reported to public health authorities in most countries, including Korea. Therefore, precise incidence and prevalence data are not available in Korea, although many reports have suggested that the number of patients with NTM lung disease is rapidly increasing in Korea.
The detection of TB cases is based primarily on the microscopic examination of sputum for AFB. This is the simplest and most efficient way to detect sources of transmission. Therefore, patient with AFB-positive sputum is diagnosed to be "bacteriologically confirmed TB case" (previously as "definite TB case")12. However, smear microscopy cannot differentiate between MTB and NTM, which causes a diagnostic dilemma. The recovery rate of NTM from AFB smear-positive sputum specimens is steadily increasing in Korea. In 255 public health centers in Korea, this rate was 6.0% in 2005 and increased to 10.3% in 2011 (Chang-Ki Kim, Korean Institute of Tuberculosis, personal communication). Moreover, this rate is dramatically rising at some referral hospitals in Korea, being 9-12% in 2001-2002 and 45-64% in 2010-201113,14,15.
The proportion of NTM among positive mycobacterial cultures is also increasing in Korea. Before the early 1990s, 97-98% of clinical isolates from sputum specimens contained MTB16. However, after the late 1990s, NTM was isolated from 20-30% of all clinical specimens submitted to mycobacterial laboratory in some referral hospitals16. At some referral hospitals, this rate further increased from about 25-40% in 2001-2002 to about 50-70% in 2010-201114,15,17,18.
The distribution of NTM species varies markedly based on geography3,4. As in other countries, Mycobacterium avium complex is the most common etiologic organism (48-76%) of NTM lung disease in Korea17,18,19. In the past, M. intracellulare was more prevalent than M. avium19,20, but now M. avium is more frequently isolated18,21,22,23. M. abscessus complex (MABC) is the second most common etiologic organism (14-33%) of NTM lung disease in Korea17,18,19,24. Within MABC, M. abscessus (44-53%) and M. massiliense (45-55%) are equally distributed, while M. bolletii is a relatively rare pathogen (1-2%) in Korea25,26,27. In addition, M. kansasii is infrequently isolated and a relatively uncommon cause of NTM lung disease in Korea28,29.
The classic clinical manifestations of pulmonary TB are chronic cough, sputum production, appetite loss, weight loss, fever, night sweats, and hemoptysis30. However, these non-specific symptoms cannot be used to differentiate between pulmonary TB and NTM lung disease. Moreover, elderly patients with pulmonary TB may have different clinical presentations than younger patients, including more frequent dyspnea and comorbid medical conditions such as cardiovascular disease, diabetes mellitus, and chronic obstructive pulmonary disease31. Previous studies have suggested that some clinical and radiographic characteristics, such as advanced age, history of not smoking, previous TB treatment, absence of pleural effusion, and involvement of middle and/or lower lung zones, are more frequently found in patients with NTM lung disease than those with pulmonary TB7,8. However, there is considerable overlap in the clinical and radiographic features of pulmonary TB and NTM lung disease7,8.
In radiographic manifestations of pulmonary TB in adults, focal or patchy heterogeneous consolidation in the apical and posterior segments of the upper lobes and the superior segments of the lower lobes were most common32. Poorly defined nodules, linear opacities, and cavities are also common findings of pulmonary TB32. In high-resolution computed tomography (HRCT), the most common lesions are centrilobular nodules, tree-in-bud lesions, patchy or lobular consolidations, and cavities32.
NTM lung disease has two different radiographic manifestations: fibrocavitary and nodular bronchiectatic forms1. Fibrocavitary forms of NTM lung disease have cavitary lesions that mostly involve the upper lobes and are similar radiographic features of pulmonary TB1. This type of disease usually develops in older males with underlying lung disease, such as previous TB (Figure 1).
Alternatively, NTM lung disease can present with nodular infiltrates that frequently involve the right middle lobe or lingular segment of the left upper lobe (Figure 2). This form of disease is termed nodular bronchiectatic disease, and it occurs predominantly in postmenopausal, non-smoking females and tends to have a much slower progression than cavitary disease1. The nodular bronchiectatic form of NTM lung disease has unique body morphotypes including lower body mass index and body fat, increased height, and increased incidence of scoliosis and pectus excavatum than control subjects33,34. Similar clinical manifestations have also been found in Korean patients35. Although bilateral bronchiectasis and bronchiolitis at HRCT also suggested NTM lung disease, there is considerable overlap in the radiographic manifestations of pulmonary TB and NTM lung disease, and radiographic findings alone could not differentiate the two diseases36.
To make a diagnosis of NTM lung disease, symptomatic patients with compatible radiographic and HRCT findings should also meet the microbiologic criteria set forth by the American Thoracic Society (ATS)/Infectious Diseases Society of America (IDSA): 1) two positive sputum culture results, or 2) one positive bronchial wash or lavage culture result, or 3) a transbronchial or other lung biopsy yielding positive culture for NTM or compatible histopathological features, such as granulomatous inflammation or stainable AFB, and one positive sputum or bronchial wash culture for NTM1.
In general, AFB staining and culture techniques adopted for MTB are satisfactory for NTM. Smear microscopy cannot differentiate between MTB and NTM. The introduction of nucleic acid amplification (NAA) tests for the detection of MTB directly from clinical specimens has greatly improved TB diagnostics. Currently, many commercial assays use different molecular approaches to amplify and detect MTB. Such tests can confirm the diagnosis of TB more reliably and with higher sensitivity than smear microscopy. Moreover, their high positive predictive values allow discrimination between MTB and NTM in smear positive specimens. If the AFB smear result is positive and the NAA result is negative, the patient can be presumed to have NTM lung disease37. Therefore, NAA tests could be used as an additional test for patients with AFB smear-positive sputum for the rapid detection of MTB and differentiation from NTM.
Ultimately, the diagnosis of NTM lung disease is based on isolation of the organism from respiratory specimens, using solid and liquid media1. The introduction of liquid culture methods has allowed for sensitive detection of both MTB and NTM38. The increased recovery rate is more prominent for NTM than MTB, especially in AFB smear-negative respiratory specimens, when liquid culture methods are combined with solid medium for mycobacterial culture39.
Rapid and accurate identification of MTB and differentiation from NTM in positive culture isolates is essential, particularly because liquid culture is associated with isolation of more NTM39. Commercial MPT64-based immunochromatographic tests and NAA tests are used for this purpose in clinical laboratories in Korea40,41.
Correct identification of species is very important because NTM species differ in their clinical relevance. The methods of identification of mycobacteria in clinical laboratories have changed dramatically over the past two decades. Molecular methods have now surpassed biochemical tests and high-performance liquid chromatography as the method of choice for identifying NTM42,43. Among molecular methods, polymerase chain reaction and restriction fragment length polymorphism analysis using the rpoB gene has been developed for use in clinical practice in Korea44,45. For precise identification of MABC at the subspecies level, as well as uncommonly encountered species, sequencing of some genes including hsp65, rpoB, and the 16S-23S rRNA internal transcribed spacer region is needed46,47,48,49,50,51.
The recovery rate of NTM from respiratory specimens and the number of patients with NTM lung disease has been rapidly increasing in Korea. An early differential diagnosis of NTM lung disease from pulmonary TB is important, as the therapeutic regimen differs from that of pulmonary TB and it is not necessary to track the contacts of patients with NTM lung disease. However, it is not always easy to distinguish NTM lung disease from pulmonary TB, because the clinical and radiographic presentations of the two diseases are similar. As symptoms, radiographic findings, and direct microscopy of respiratory specimens are insufficient to differentiate between pulmonary TB and NTM lung disease, mycobacterial culture and correct identification remain the gold standard.
Acknowledgements
This study was supported by Samsung Biomedical Research Institute grant [SMO1131811].
References
1. Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 2007;175:367-416. PMID: 17277290.


2. Daley CL, Griffith DE. Pulmonary non-tuberculous mycobacterial infections. Int J Tuberc Lung Dis 2010;14:665-671. PMID: 20487602.

3. Kendall BA, Winthrop KL. Update on the epidemiology of pulmonary nontuberculous mycobacterial infections. Semin Respir Crit Care Med 2013;34:87-94. PMID: 23460008.



4. Hoefsloot W, van Ingen J, Andrejak C, Angeby K, Bauriaud R, Bemer P, et al. The geographic diversity of nontuberculous mycobacteria isolated from pulmonary samples: an NTM-NET collaborative study. Eur Respir J 2013;42:1604-1613. PMID: 23598956.


5. Maiga M, Siddiqui S, Diallo S, Diarra B, Traore B, Shea YR, et al. Failure to recognize nontuberculous mycobacteria leads to misdiagnosis of chronic pulmonary tuberculosis. PLoS One 2012;7:e36902PMID: 22615839.



6. Kang BJ, Jo KW, Park TS, Yoo JW, Lee SW, Choi CM, et al. Causes and predictive factors associated with "diagnosis changed" outcomes in patients notified as tuberculosis cases in a private tertiary hospital. Tuberc Respir Dis 2013;75:238-243.

7. Koh WJ, Yu CM, Suh GY, Chung MP, Kim H, Kwon OJ, et al. Pulmonary TB and NTM lung disease: comparison of characteristics in patients with AFB smear-positive sputum. Int J Tuberc Lung Dis 2006;10:1001-1007. PMID: 16964791.

8. Kendall BA, Varley CD, Choi D, Cassidy PM, Hedberg K, Ware MA, et al. Distinguishing tuberculosis from nontuberculous mycobacteria lung disease, Oregon, USA. Emerg Infect Dis 2011;17:506-509. PMID: 21392445.



9. Hong YP, Kim SJ, Lew WJ, Lee EK, Han YC. The seventh nationwide tuberculosis prevalence survey in Korea, 1995. Int J Tuberc Lung Dis 1998;2:27-36. PMID: 9562108.

10. Park YK, Park YS, Na KI, Cho EH, Shin SS, Kim HJ. Increased tuberculosis burden due to demographic transition in Korea from 2001 to 2010. Tuberc Respir Dis 2013;74:104-110.

11. Korea Centers for Disease Control and Prevention. Annual report on the notified tuberculosis in Korea. Cheonju: Korea Centers for Disease Control and Prevention; 2013.
12. World Health Organization. Definitions and reporting framework for tuberculosis - 2013 revision [Internet]. Geneva: World Health Organization; 2013. cited 2014 Apr 1. Available from: http://apps.who.int/iris/bitstream/10665/79199/1/9789241505345_eng.pdf.
13. Jeon K, Koh WJ, Kwon OJ, Suh GY, Chung MP, Kim H, et al. Recovery rate of NTM from AFB smear-positive sputum specimens at a medical centre in South Korea. Int J Tuberc Lung Dis 2005;9:1046-1051. PMID: 16158899.

14. Yoo JW, Jo KW, Kim MN, Lee SD, Kim WS, Kim DS, et al. Increasing trend of isolation of non-tuberculous mycobacteria in a tertiary university hospital in South Korea. Tuberc Respir Dis 2012;72:409-415.

15. Koh WJ, Chang B, Jeong BH, Jeon K, Kim SY, Lee NY, et al. Increasing Recovery of nontuberculous mycobacteria from respiratory specimens over a 10-year period in a tertiary referral hospital in South Korea. Tuberc Respir Dis 2013;75:199-204.

16. Koh WJ, Kwon OJ, Lee KS. Diagnosis and treatment of nontuberculous mycobacterial pulmonary diseases: a Korean perspective. J Korean Med Sci 2005;20:913-925. PMID: 16361797.



17. Park YS, Lee CH, Lee SM, Yang SC, Yoo CG, Kim YW, et al. Rapid increase of non-tuberculous mycobacterial lung diseases at a tertiary referral hospital in South Korea. Int J Tuberc Lung Dis 2010;14:1069-1071. PMID: 20626955.

18. Lee SK, Lee EJ, Kim SK, Chang J, Jeong SH, Kang YA. Changing epidemiology of nontuberculous mycobacterial lung disease in South Korea. Scand J Infect Dis 2012;44:733-738. PMID: 22720876.


19. Koh WJ, Kwon OJ, Jeon K, Kim TS, Lee KS, Park YK, et al. Clinical significance of nontuberculous mycobacteria isolated from respiratory specimens in Korea. Chest 2006;129:341-348. PMID: 16478850.


20. Ryoo SW, Shin S, Shim MS, Park YS, Lew WJ, Park SN, et al. Spread of nontuberculous mycobacteria from 1993 to 2006 in Koreans. J Clin Lab Anal 2008;22:415-420. PMID: 19021271.



21. Koh WJ, Jeong BH, Jeon K, Lee NY, Lee KS, Woo SY, et al. Clinical significance of the differentiation between Mycobacterium avium and Mycobacterium intracellulare in M. avium complex lung disease. Chest 2012;142:1482-1488. PMID: 22628488.


22. Kim HS, Lee Y, Lee S, Kim YA, Sun YK. Recent trends in clinically significant nontuberculous mycobacteria isolates at a Korean general hospital. Ann Lab Med 2014;34:56-59. PMID: 24422197.


23. Jang MA, Koh WJ, Huh HJ, Kim SY, Jeon K, Ki CS, et al. Distribution of nontuberculous mycobacteria by multigene sequence-based typing and clinical significance of isolated strains. J Clin Microbiol 2014;52:1207-1212. PMID: 24501029.



24. Jeon K, Kwon OJ, Lee NY, Kim BJ, Kook YH, Lee SH, et al. Antibiotic treatment of Mycobacterium abscessus lung disease: a retrospective analysis of 65 patients. Am J Respir Crit Care Med 2009;180:896-902. PMID: 19661243.


25. Kim HY, Kook Y, Yun YJ, Park CG, Lee NY, Shim TS, et al. Proportions of Mycobacterium massiliense and Mycobacterium bolletii strains among Korean Mycobacterium chelonae-Mycobacterium abscessus group isolates. J Clin Microbiol 2008;46:3384-3390. PMID: 18753344.



26. Koh WJ, Jeon K, Lee NY, Kim BJ, Kook YH, Lee SH, et al. Clinical significance of differentiation of Mycobacterium massiliense from Mycobacterium abscessus. Am J Respir Crit Care Med 2011;183:405-410. PMID: 20833823.


27. Lee SH, Yoo HK, Kim SH, Koh WJ, Kim CK, Park YK, et al. The drug resistance profile of Mycobacterium abscessus group strains from Korea. Ann Lab Med 2014;34:31-37. PMID: 24422193.


28. Yim JJ, Park YK, Lew WJ, Bai GH, Han SK, Shim YS. Mycobacterium kansasii pulmonary diseases in Korea. J Korean Med Sci 2005;20:957-960. PMID: 16361804.



29. Park HK, Koh WJ, Shim TS, Kwon OJ. Clinical characteristics and treatment outcomes of Mycobacterium kansasii lung disease in Korea. Yonsei Med J 2010;51:552-556. PMID: 20499421.



30. Zumla A, Raviglione M, Hafner R, von Reyn CF. Tuberculosis. N Engl J Med 2013;368:745-755. PMID: 23425167.


31. Kwon YS, Chi SY, Oh IJ, Kim KS, Kim YI, Lim SC, et al. Clinical characteristics and treatment outcomes of tuberculosis in the elderly: a case control study. BMC Infect Dis 2013;13:121PMID: 23510403.




32. Jeong YJ, Lee KS. Pulmonary tuberculosis: up-to-date imaging and management. AJR Am J Roentgenol 2008;191:834-844. PMID: 18716117.


33. Kim RD, Greenberg DE, Ehrmantraut ME, Guide SV, Ding L, Shea Y, et al. Pulmonary nontuberculous mycobacterial disease: prospective study of a distinct preexisting syndrome. Am J Respir Crit Care Med 2008;178:1066-1074. PMID: 18703788.



34. Kartalija M, Ovrutsky AR, Bryan CL, Pott GB, Fantuzzi G, Thomas J, et al. Patients with nontuberculous mycobacterial lung disease exhibit unique body and immune phenotypes. Am J Respir Crit Care Med 2013;187:197-205. PMID: 23144328.



35. Lee AR, Lee J, Choi SM, Seong MW, Kim SA, Kim M, et al. Phenotypic, immunologic, and clinical characteristics of patients with nontuberculous mycobacterial lung disease in Korea. BMC Infect Dis 2013;13:558PMID: 24274658.




36. Koh WJ, Lee KS, Kwon OJ, Jeong YJ, Kwak SH, Kim TS. Bilateral bronchiectasis and bronchiolitis at thin-section CT: diagnostic implications in nontuberculous mycobacterial pulmonary infection. Radiology 2005;235:282-288. PMID: 15703315.


37. Centers for Disease Control and Prevention (CDC). Updated guidelines for the use of nucleic acid amplification tests in the diagnosis of tuberculosis. MMWR Morb Mortal Wkly Rep 2009;58:7-10. PMID: 19145221.


38. Koh WJ, Ko Y, Kim CK, Park KS, Lee NY. Rapid diagnosis of tuberculosis and multidrug resistance using a MGIT 960 system. Ann Lab Med 2012;32:264-269. PMID: 22779067.



39. Chihota VN, Grant AD, Fielding K, Ndibongo B, van Zyl A, Muirhead D, et al. Liquid vs. solid culture for tuberculosis: performance and cost in a resource-constrained setting. Int J Tuberc Lung Dis 2010;14:1024-1031. PMID: 20626948.

40. Park MY, Kim YJ, Hwang SH, Kim HH, Lee EY, Jeong SH, et al. Evaluation of an immunochromatographic assay kit for rapid identification of Mycobacterium tuberculosis complex in clinical isolates. J Clin Microbiol 2009;47:481-484. PMID: 19052177.


41. Park KS, Kim JY, Lee JW, Hwang YY, Jeon K, Koh WJ, et al. Comparison of the Xpert MTB/RIF and Cobas TaqMan MTB assays for detection of Mycobacterium tuberculosis in respiratory specimens. J Clin Microbiol 2013;51:3225-3227. PMID: 23863563.



42. van Ingen J. Diagnosis of nontuberculous mycobacterial infections. Semin Respir Crit Care Med 2013;34:103-109. PMID: 23460010.



43. Chihota VN, van Halsema CL, Grant AD, Fielding KL, van Helden PD, Churchyard GJ, et al. Spectrum of non-tuberculous mycobacteria identified using standard biochemical testing vs. 16S sequencing. Int J Tuberc Lung Dis 2013;17:267-269. PMID: 23228479.


44. Lee H, Bang HE, Bai GH, Cho SN. Novel polymorphic region of the rpoB gene containing Mycobacterium species-specific sequences and its use in identification of mycobacteria. J Clin Microbiol 2003;41:2213-2218. PMID: 12734283.



45. Kim S, Park EM, Kwon OJ, Lee JH, Ki CS, Lee NY, et al. Direct application of the PCR restriction analysis method for identifying NTM species in AFB smear-positive respiratory specimens. Int J Tuberc Lung Dis 2008;12:1344-1346. PMID: 18926049.

46. Choi GE, Chang CL, Whang J, Kim HJ, Kwon OJ, Koh WJ, et al. Efficient differentiation of Mycobacterium abscessus complex isolates to the species level by a novel PCR-based variable-number tandem-repeat assay. J Clin Microbiol 2011;49:1107-1109. PMID: 21177894.



47. Shin SJ, Choi GE, Cho SN, Woo SY, Jeong BH, Jeon K, et al. Mycobacterial genotypes are associated with clinical manifestation and progression of lung disease caused by Mycobacterium abscessus and Mycobacterium massiliense. Clin Infect Dis 2013;57:32-39. PMID: 23511298.



48. Jeong BH, Song JU, Kim W, Han SG, Ko Y, Song J, et al. Nontuberculous mycobacterial lung disease caused by Mycobacterium lentiflavum in a patient with bronchiectasis. Tuberc Respir Dis 2013;74:187-190.

49. Jeong BH, Kim SY, Jeon K, Huh HJ, Ki CS, Lee NY, et al. The first Korean case of nontuberculous mycobacterial lung disease caused by Mycobacterium abscessus subspecies bolletii in a patient with bronchiectasis. Tuberc Respir Dis 2014;76:30-33.

50. Kim SY, Shin SJ, Lee NY, Koh WJ. First case of pulmonary disease caused by a Mycobacterium avium complex strain of presumed veterinary origin in an adult human patient. J Clin Microbiol 2013;51:1993-1995. PMID: 23554206.



51. Kim SY, Yoo H, Jeong BH, Jeon K, Ha YE, Huh HJ, et al. First case of nontuberculous mycobacterial lung disease caused by Mycobacterium marseillense in a patient with systemic lupus erythematosus. Diagn Microbiol Infect Dis 2014;79:355-357. PMID: 24768296.


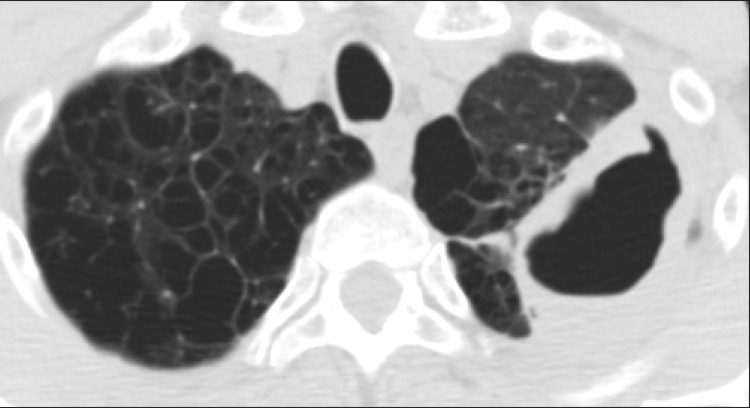
Figure┬Ā1
Fibrocavitary form of nontuberculous mycobacterial lung disease in a 53-year-old male with Mycobacterium avium lung disease. Chest high-resolution computed tomography shows a large cavity in the left upper lobe. Note the severe emphysema in both lungs.
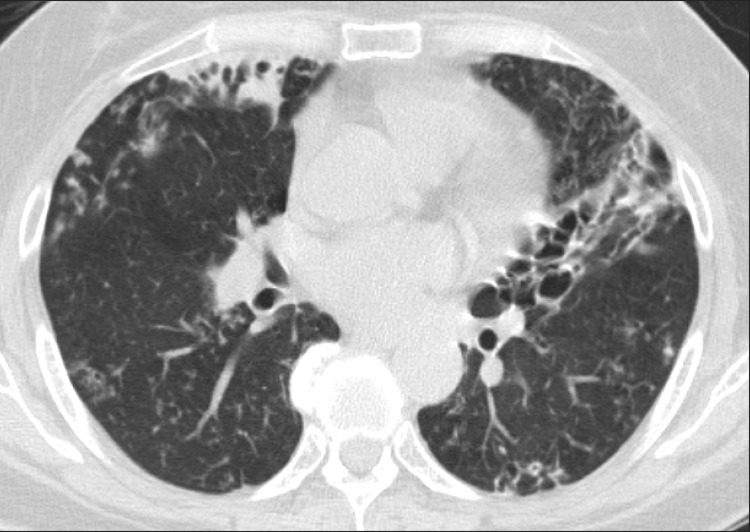
Figure┬Ā2
Nodular bronchiectatic form of nontuberculous mycobacterial lung disease in a 60-year-old female with Mycobacterium abscessus lung disease. Chest high-resolution computed tomography shows severe bronchiectasis in the right middle lobe and the lingular segment of the left upper lobe. Note the multiple small nodules suggesting bronchiolitis in both lungs.


 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Print
Print Download Citation
Download Citation




