Introduction
Tuberculosis is a significant infectious problem in elderly patients with comorbidities in Korea
1. The incidence of tuberculosis is increasing in older patients, and some have multiple comorbidities. This review summarizes the treatment of tuberculosis in patients with comorbidities including hepatic disease, chronic kidney disease, solid-organ transplantation, and solid malignant disease. This review is based on the Korean Guidelines for Tuberculosis, first edition, 2011
2.
Hepatic Disease
Drug-induced liver injury (DILI) is a problem associated with the treatment of tuberculosis. Chronic liver disease is known to increase the risk of DILI. DILI in patients with advanced liver disease is potentially serious, even life-threatening
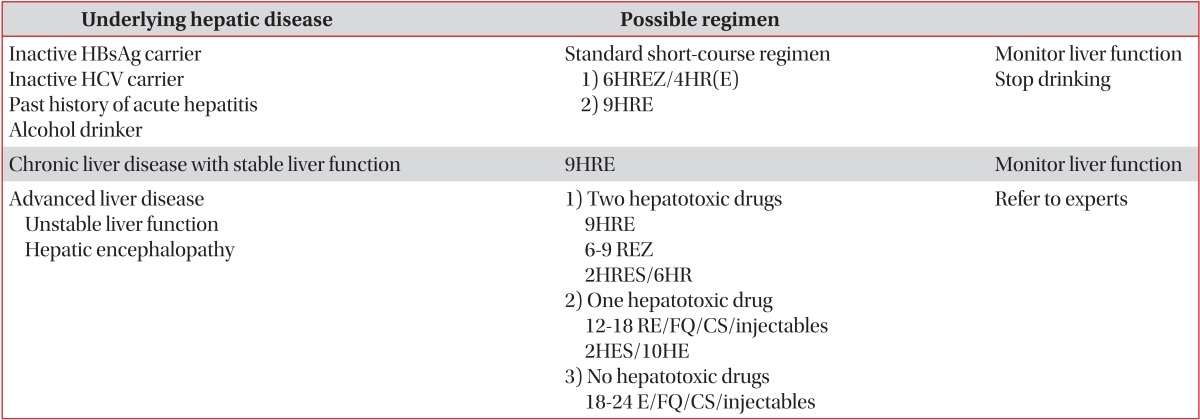
3. Thus, clinicians must carefully monitor hepatic function in patients with liver disease during the treatment of tuberculosis. The choice of the treatment regimen used in the setting of liver disease depends on the severity of both the liver disease and the tuberculosis (
Table 1)
2,3,4.
Lee et al.
5 reported that tuberculosis treatment in hepatitis B surface antigen (HBsAg)-positive inactive carriers can be performed in the usual manner, using standard short-course regimens of isoniazid, rifampin, ethambutol, and/or pyrazinamide with careful monthly monitoring of liver function. In that study, the authors showed that DILI occurred more frequently in HBsAg carriers (9 of 110 carriers, 8%) than in control subjects (4 of 97 controls, 4%). However, after the recovery of liver function, isoniazid and rifampin were successfully reintroduced as therapy. Park et al.
6 reported on the treatment of tuberculosis in patients with chronic hepatitis and cirrhosis. In that study, the authors showed that hepatotoxic antituberculosis drugs might be safely used in patients with chronic liver disease, including compensated cirrhosis, if the number of hepatotoxic drugs used was appropriately adjusted.
Renal Insufficiency and End-Stage Renal Disease
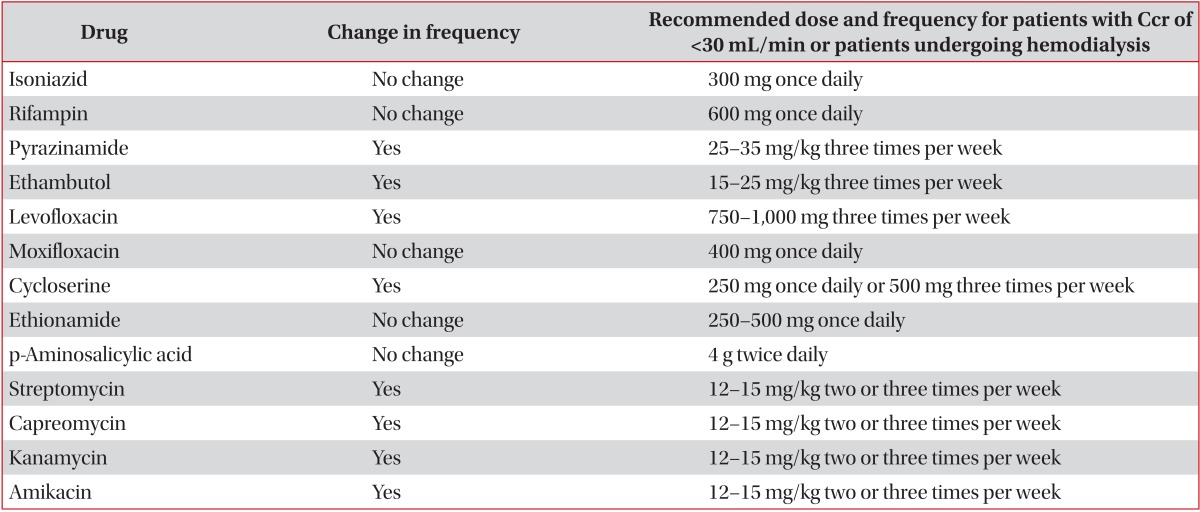
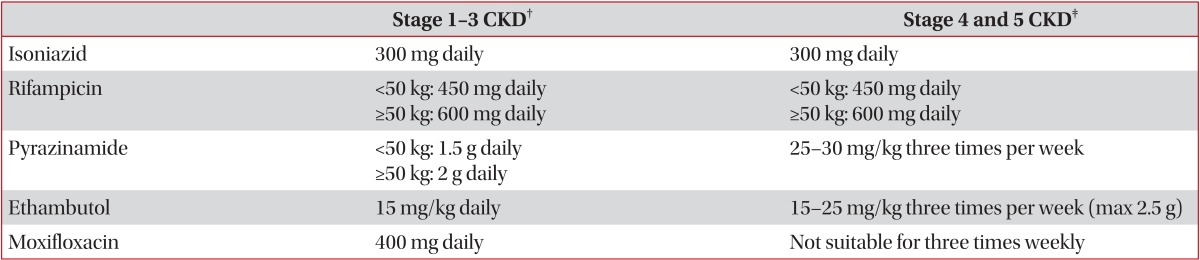
Renal insufficiency complicates the treatment of tuberculosis because some antituberculosis drugs are cleared by the kidney. Management may be further complicated by the removal of some antituberculosis drugs via hemodialysis
3. Thus, clinicians should carefully monitor the potential development of drug-induced toxicity and the efficacy of antituberculosis treatment in patients with renal insufficiency. Some antituberculosis drugs require adjustment of their dosing and administration time intervals. Vitamin B6 should be given to these patients to prevent peripheral neuropathy due to isoniazid. The recommendations for adjustment of antituberculosis drug are shown in
Table 2,2,3 and
Table 3,7.
Solid-Organ Transplant
The risk for tuberculosis in patients with solid-organ transplantation is estimated to be 20 to 74 times greater than that in the general population
8. Because of the challenge of treating active tuberculosis after transplantation, every effort must be made to diagnose and treat active tuberculosis before transplantation. The standard short-course treatment for active tuberculosis is recommended in patients undergoing transplantation
8. The major difficulty in administering antituberculosis drugs to transplant patients is drug-drug interactions involving rifampin. Rifampin is a strong inducer of the microsomal enzymes that metabolize cyclosporine, tacrolimus, sirolimus, and corticosteroids. It may be difficult to maintain adequate levels of immunosuppressive drugs while using rifampin. Successful use of rifampin has been reported in transplant recipients, but the doses of cyclosporine, tacrolimus, and sirolimus must be increased at least two- to five-fold
8.
Solid-Organ Malignancy
There are no specific guidelines for the treatment of tuberculosis in patients with solid-organ malignancy. Standard treatment for tuberculosis is recommended in this population. Kim et al.
9 reported that the radiographic and clinical treatment responses during anticancer chemotherapy were not clinically different between tuberculosis-infected patients with (n=24) and without (n=48) solid-organ malignancy.
Conclusion
Tuberculosis is still a significant public health concern in Korea. The growing elderly population with higher incidences of comorbidities has a risk of developing tuberculosis. Clinicians must monitor the side effects of and response to antituberculosis therapy in patients with comorbidities.





 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Print
Print Download Citation
Download Citation



