Progression-Free Survival: An Important Prognostic Marker for Long-Term Survival of Small Cell Lung Cancer
Article information
Abstract
Background
Small cell lung cancer (SCLC) is an extremely aggressive tumor with a poor clinical course. Although many efforts have been made to improve patients' survival rates, patients who survive longer than 2 years after chemotherapy are still very rare. We examined the baseline characteristics of patients with long-term survival rates in order to identify the prognostic factors for overall survivals.
Methods
A total of 242 patients with cytologically or histologically diagnosed SCLC were enrolled into this study. The patients were categorized into long- and short-term survival groups by using a survival cut-off of 2 years after diagnosis. Cox's analyses were performed to identify the independent factors.
Results
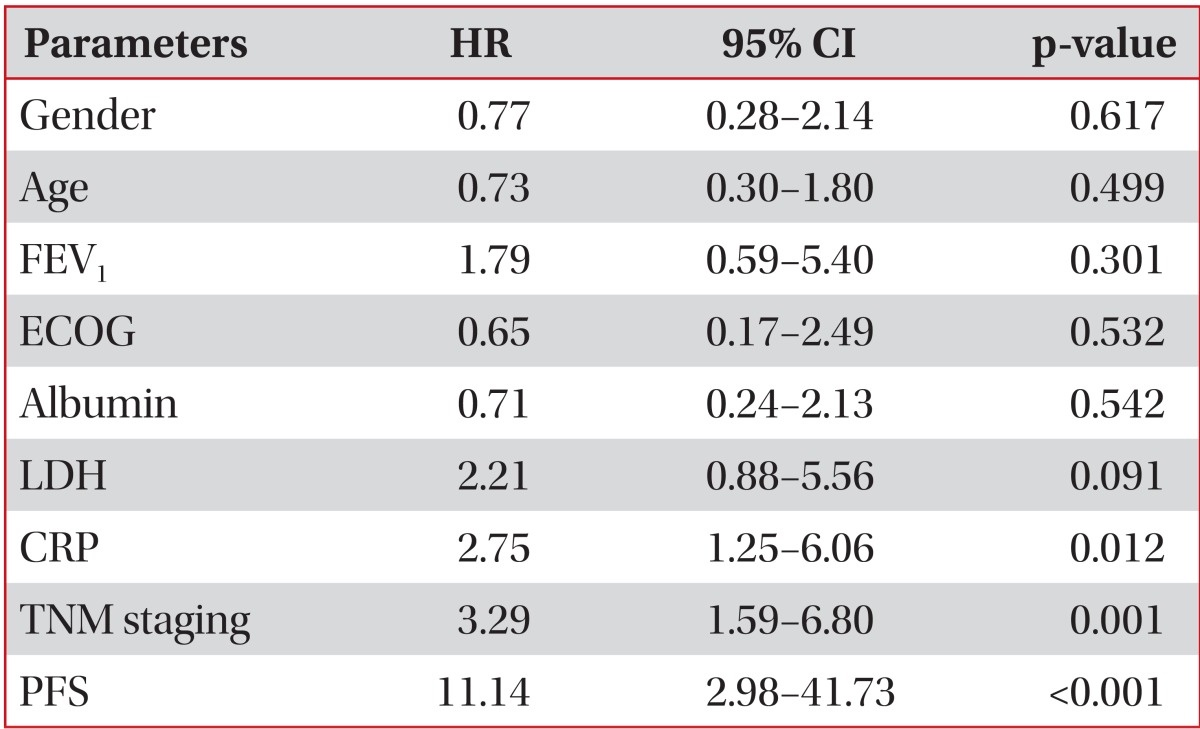
The mean patient age was 65.66 years, and 85.5% were males; among the patients, 61 of them (25.2%) survived longer than 2 years. In the multivariate analyses, CRP (hazard ratio [HR], 2.75; 95% confidence interval [CI], 1.25-6.06; p=0.012), TNM staging (HR, 3.29; 95% CI, 1.59-6.80; p=0.001), and progression-free survival (PFS) (HR, 11.14; 95% CI, 2.98-41.73; p<0.001) were independent prognostic markers for poor survival rates.
Conclusion
In addition to other well-known prognostic factors, this study discovered relationships between the long-term survival rates and serum CRP levels, TNM staging, and PFS. In situations with unfavorable conditions, the PFS would be particularly helpful for managing SCLC patients.
Introduction
Small cell lung cancer (SCLC) is an extremely aggressive tumor with a poor clinical course. Without treatment, the median length of survival after diagnosis is 1-3 months. Despite the initially high response rate to chemotherapy, the overall survival (OS) rate is low, with only 10-15% of patients with limited stage (LS)- or extensive stage (ES)-SCLC alive 2 years after diagnosis1. The median survival time for patients with ES-SCLC is 12-20 months, depending on the disease stage1.
Causes of the poor outcome of SCLC have been described, including more rapid tumor doubling times, a higher growth fraction, and earlier development of widespread metastases compared to non-small cell lung cancer (NSCLC). The tumor doubling time of primary lung cancer is known to be a significant prognostic factor for lung cancer patients2. A short doubling time is the most reliable risk factor for multiple metastases, early recurrence, and a poor prognosis3. SCLC is considered the prototype of rapid-growing malignancies, with doubling times in the range of 25-217 days according to several studies4,5,6.
Although the results have been inconsistent, prior studies have identified a number of prognostic factors for SCLC7,8,9. Those factors, including age, sex, smoking history, syndrome of inappropriate secretion of antidiuretic hormone (SIADH), platelet count, and levels of albumin, lactate dehydrogenase (LDH), neuron-specific enolase (NSE), carcinoembryonic antigen, and cytokeratin-19 fragment, were determined to be potential prognostic factors for survival8,9,10. The International Association for the Study of Lung Cancer (IASLC) staging system has been used as a prognostic factor because it was found to be better than the Veterans Administration Lung Study Group (VALG) staging system for estimating long-term survival in patients with SCLC11. National Comprehensive Cancer Network (NCCN) 2014 guidelines (version 2) suggested that performance status, VALG staging, weight loss, and serum LDH level were prognostic factors for SCLC. Additionally, depending on the VALG stage, sex, age, serum creatinine level, and the number of metastatic organs at the time of diagnosis could be prognostic factors12.
Despite increased effort to find prognostic factors for SCLC, we could not find consistent and meaningful markers. In this study, we investigated prognostic factors that are related with treatment strategy, new drug targets, and clinical trials.
Materials and Methods
1. Data collection
This retrospective study included patients who were cytologically or histologically diagnosed with SCLC at Chungnam National University Hospital (Daejeon, Korea) between January 2003 and July 2009. Medical records, as well as radiologic and nuclear medicine reports, were reviewed. Additionally, data were collected regarding the patients' age, physical measurements, smoking history, pulmonary function, performance status, stage, and levels of serum albumin, LDH, and C-reactive protein (CRP) at the time of diagnosis. Data regarding the patients' sex, existence of prophylactic cranial irradiation (PCI), progression-free survival (PFS), survival post-progression (SPP), response to initial treatment, and total survival length were also collected. Patients with lost to follow-up within 2 years or active concurrent infection were excluded.
2. Clinical variables
The final follow-up date was 31 July 2011. The patients were categorized into two groups according to the duration of survival after diagnosis: >2 years or <2 years. Patients who survived >2 years after they were first diagnosed with SCLC were defined as long-term survivors. Physical measurement data included height, body weight, and body mass index (BMI). We used the pack/year unit for patients with a history of smoking before the diagnosis of SCLC, regardless of whether they had stopped smoking. Pulmonary function was evaluated spirometrically using the forced expiratory volume in 1 second (FEV1), which was performed within 2 months of the initial diagnosis. Performance status was defined using the Eastern Cooperative Oncology Group (ECOG) scale (stages 0-3) at the time of diagnosis. Two staging systems, the VALG system, which has been used only in SCLC, and the IASLC system (American Joint Committee on Cancer [AJCC] 7th edition), which has been used both in SCLC and NSCLC, were used to estimate stage based on chest computed tomography (CT), positron emission tomography-CT (PET-CT), brain CT, and brain magnetic resonance imaging. We used the highest levels of serum albumin, LDH, and CRP obtained between the date of SCLC diagnosis and that of initial treatment. After initial diagnosis, patients received treatment for SCLC with one of the 5 types of treatment arm which consist of chemotherapy, concurrent chemoradiotherapy, operation, radiotherapy, supportive care. Response evaluation using chest CT scan was performed after every 2-3 cycles of chemotherapy. Patients who received radiotherapy or operation, chest CT scan was conducted 4 weeks after the initial treatment. After the treatment, chest CT scan was recommended every 2 months for the first year, 3 months for the second year, every 6 months for the next several years, at least yearly after 5 years. Data for the treatment response, which was based on Response Evaluation Criteria in Solid Tumors from chest CT or PET-CT findings after initial treatment, were collected, excluding data of supportive care. In the case of disease progression, subsequent chemotherapy with single agent (i.e., IV topotecan) was conducted in favorable performance status. PFS was defined as the period from the time of initial treatment until an increased size of the primary mass or new lesion was found on radiologic or nuclear medicine examination. PFS was not measured in patients who underwent supportive care. SPP was calculated by subtracting OS from PFS.
3. Statistical analyses
All analyses were performed using PASW Statistics (version 18.0; SPSS Inc., Chicago, IL, USA). Independent t-tests were used to assess relationships between the existence of long-term survival and continuous variables such as age, physical measurements, smoking history, FEV1, PFS, and serum albumin, LDH, and CRP levels. Chi-squared tests were used to assess the relationship between long-term survival and categorical variables such as sex, performance status, stage, conduction of PCI, and treatment response, as well as some continuous variables classified as categorical, including age, FEV1, and serum creatinine, albumin, LDH, and CRP levels. In addition, for variables identified as having a significant effect on long-term survival, the Kaplan-Meier method was used to determine survival curves. Significant differences between survival curves were estimated using log-rank tests. To identify prognostic factors, multivariate analyses using Cox's regression analysis were conducted.
4. Ethical considerations
The Institutional Review Board at Chungnam National University Hospital reviewed and approved the study protocol (project approval number: CNUH 2012-07-006-001).
Results
1. Patient information
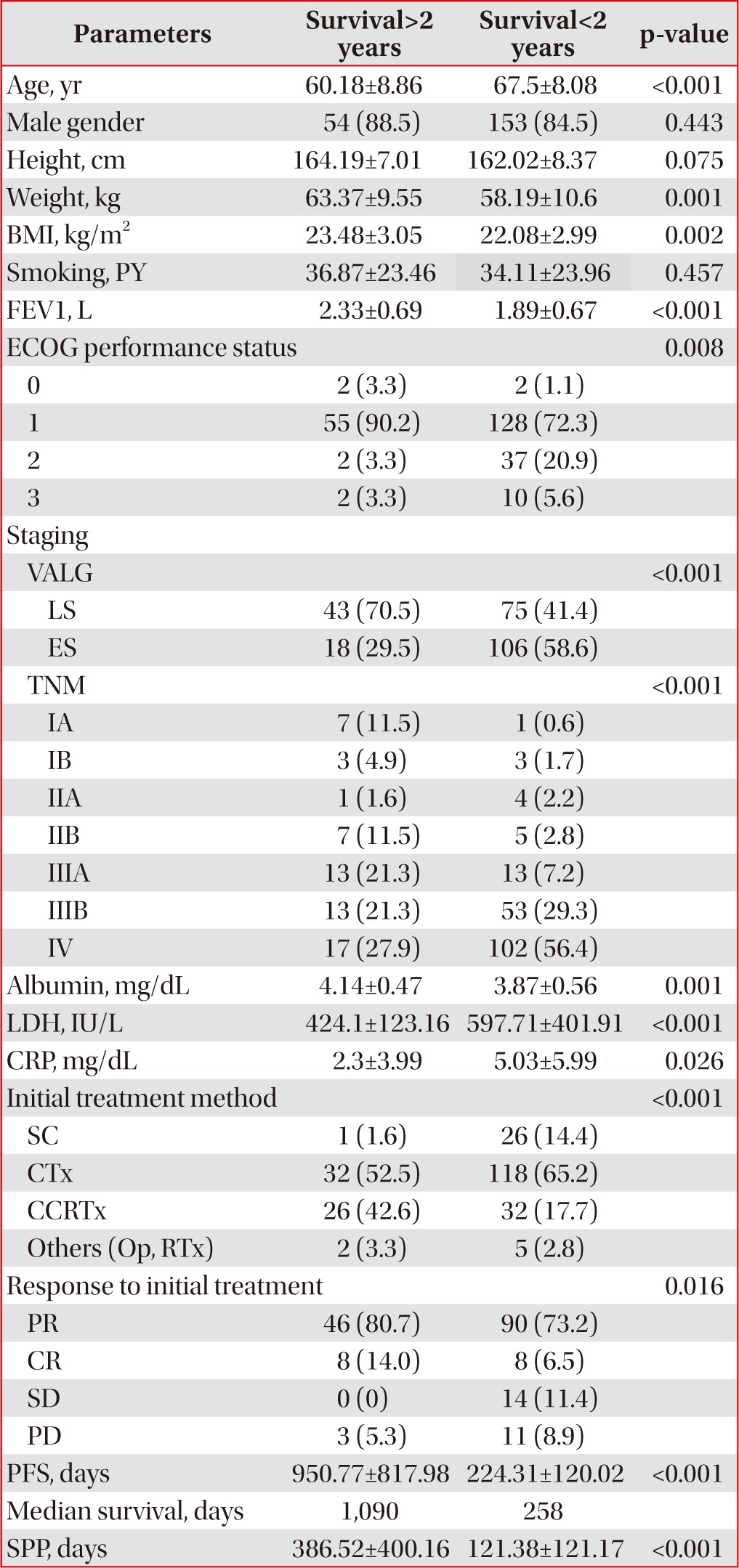
Two hundred and forty-two patients participated in the present study. The mean patient age was 65.66 years, and 85.5% of the patients were male. The median survival length was 349.5 days; the maximum was 2,961 days. Sixty-one (25.2%) patients were long-term (>2 years) survivors. Many variables had missing data except for age, sex, stage, initial treatment method, and survival length factors, with CRP level demonstrating the most missing patient data (n=139). The patients' baseline characteristics are described in Table 1.
2. Relationship between long-term survival and other factors
In an analysis of the relationship between long-term survival and factors for which means could be calculated, height (p=0.075) and smoking history (p=0.457) were not significant. However, factors such as mean age, weight, BMI, FEV1, PFS, albumin, LDH, and CRP levels differed significantly according to long-term survival (Table 1).
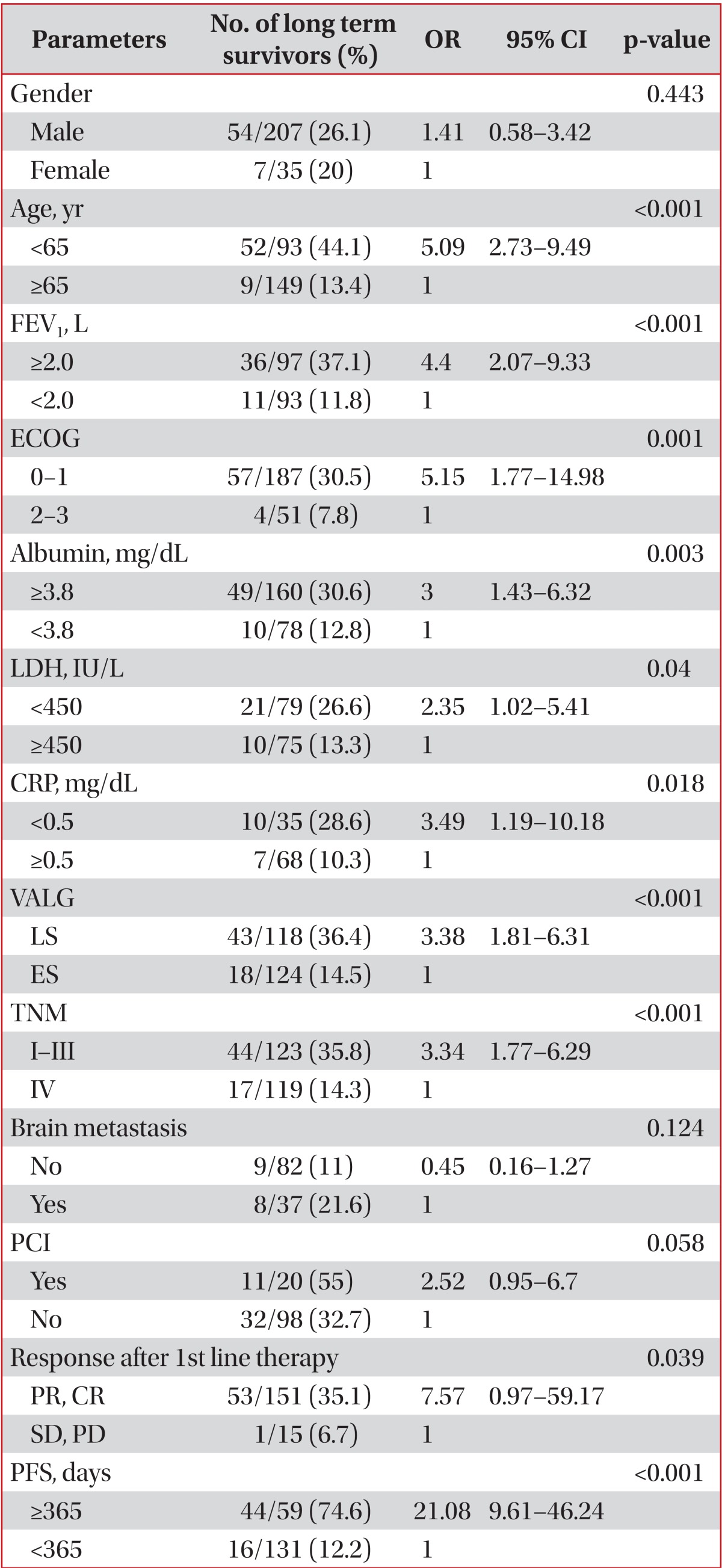
More men (26.1%) than women (20%) survived >2 years, but sex was not significantly related to long-term survival with SCLC (odds ratio [OR], 1.41; 95% confidence interval [CI], 0.58-3.42; p=0.443). Age <65 years was related to long-term survival (OR, 5.09; 95% CI, 2.73-9.49; p<0.001), as were ECOG performance statuses 0 and 1 (OR, 5.52; 95% CI, 1.77-14.98; p=0.001). Concerning VALG stage, LS was related to long-term survival (OR, 3.376; 95% CI, 1.81-6.31; p<0.001), and metastasis group based on TNM staging showed a similar relationship (OR, 3.34; 95% CI, 1.77-6.29; p<0.001). However, the absence of brain metastasis also showed no relationship to long-term survival (OR, 0.447; 95% CI, 0.16-1.27; p=0.124). An FEV1≥2.0 L was related to long-term survival (OR, 4.40; 95% CI, 2.07-9.33; p<0.001), as were an albumin level≥3.8 mg/dL, LDH level<450 IU/L, and CRP level<0.5 mg/dL (ORs, 3.00, 2.35, and 3.49, respectively; 95% CIs, 1.43-6.32, 1.02-5.41, and 1.19-5.41, respectively; p=0.003, p=0.04, and p=0.018, respectively). Additionally, in the LS patients, performing PCI showed no significant relationship to long-term survival (OR, 2.52; 95% CI, 0.95-6.70; p=0.058). To determine the relationship between treatment response and long-term survival, we classified the initial treatment response into four categories: partial remission, complete remission, stable disease, and progressive disease. We then analyzed the relationship between the remission groups and long-term survival, which showed statistical significance (OR, 7.57; 95% CI, 0.97-59.17; p=0.039). However, when the partial remission, complete remission, and stable disease groups were designated as the control group and the progressive disease group as the non-control group, the control group had no significant relationship to long-term survival (OR, 1.77; 95% CI, 0.47-6.60; p=0.391). PFS≥365 days was related to long-term survival (OR, 21.08; 95% CI, 9.61-46.24; p<0.001). Table 2 shows the relationships between long-term survival and nominal variables.
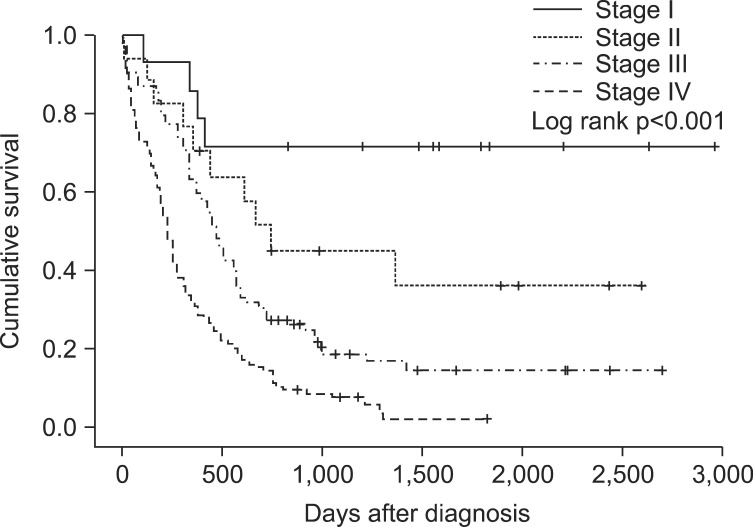
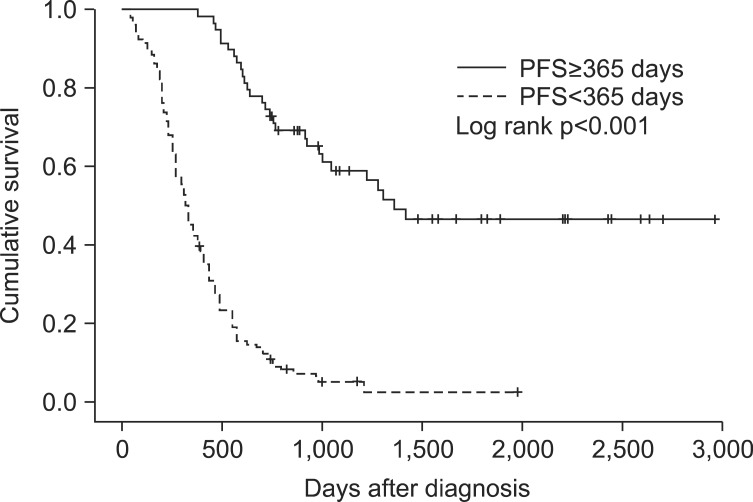
Significant differences in median OS were found for serum CRP levels, TNM staging, and PFS. The median OS for CRP<0.5 (n=35) was 408 days and for CRP≥0.5 (n=68) was 196 days (p=0.004) (Figure 1). The median OS for stage I (n=14) could not be calculated because over 50% of candidates survived, stage II (n=17) was 740 days, stage III (n=92) was 470 days, and stage IV (n=119) was 230 (p<0.001) (Figure 2). The median OS for PFS≥365 (n=59) and for PFS<365 (n=131) was 324 days (p<0.001) (Figure 3). In multivariate analyses using Cox's regression, CRP (hazard ratio [HR], 2.75; 95% CI, 1.25-6.06; p=0.012), TNM staging (HR, 3.29; 95% CI, 1.59-6.80; p=0.001), and PFS (HR, 11.14; 95% CI, 2.98-41.73; p<0.001) (Table 3) were independent prognostic markers for poor survival.
Discussion
In this study, previously known prognostic factors, including age, weight, BMI, serum albumin levels, LDH levels, performance status, and staging, were related to survival for >2 years. In particular, serum CRP levels, TNM staging and PFS were prognostic factors in SCLC patients by multivariate analyses.
The CRP test is sensitive and widely used in the diagnosis of acute and chronic inflammation. In malignant tumors, inflammatory cells produce various cytokines; particularly, interleukin (IL)-6 induces necrosis and inflammation of tumor tissue. CRP is produced by hepatocytes, the level of which is affected by IL-613. CRP is known to be a prognostic factor in many malignant tumors such as hepatocellular carcinoma, esophageal cancer, and colon cancer14,15,16. A few studies of NSCLC have also used CRP as an independent risk factor17,18. Some studies have shown that dynamic changes in the serum CRP level can be used as a predicting factor for treatment response in SCLC19,20. One recent study examined the use of pretreatment CRP level as a prognostic factor21. In the current study, the long-term survival group had a significantly lower CRP level at the time of diagnosis, which seemed valuable as a prognostic factor for SCLC; however, data were missing for 139 patients, which is the largest amount among all variables. To gain statistical reliability, a study with more subjects is needed.
The VALG system has been used for staging SCLC since 195722. Radiation therapy plays an important role in managing SCLC patients with cytotoxic chemotherapy. Thus, although the staging system is old, many treatment guidelines for SCLC are based on the VALG system, which is categorized by whether all known lesions are included in a single radiation field. However, the revised TNM staging system that was embraced in AJCC (7th edition) and was approved for prognostic significance in NSCLC as well as SCLC, is used in selecting treatment modalities for SCLC by NCCN 2014 guidelines (version 2)12,23. The fact that TNM staging system was a prognostic factor for survival in this study is appropriate based on recent trends.
The US Food and Drug Administration defines PFS as "the time from randomization until objective tumor progression or death"24. Generally, PFS is not commonly considered a prognostic factor in malignant tumors because its correlation with OS is rare. Additionally, no report to date has shown that PFS is a prognostic factor in SCLC. In this study, however, longer PFS was related to long-term survival. Recently, PFS has come to the forefront as a primary endpoint, replacing OS in clinical trials developing anticancer agents, particularly in phase II or III trials. PFS has many advantages in clinical trials compared with OS. Disease progression can be observed earlier than the expiration of candidates, leading to lighter burdens with fewer candidates and shorter trial durations. In addition, the effects of subsequent therapy (after the study period) do not interfere with PFS. Longer PFS that delays tumor-associated morbidity, including mental and physical aspects, may improve quality of life (QOL) because PFS has an intimate connection with tumor progression. Most anticancer agents that have been developed recently target angiogenesis or growth factor receptors (i.e., they are focused on cytostatic features). In the past, there was a different perspective because drugs focused on the death of tumor cells or decreased numbers of tumor cells. Therefore, in phase II or III trials, PFS that covers stable disease is preferable to an objective tumor response, which was previously used as an endpoint25. PFS, however, has limitations in replacing OS in clinical trials. Judging disease progression precisely by radiologic examination would depend on subjective interpretation. Moreover, the definitions of disease progression and time of response evaluation may be different between studies25,26.
It thus appears that PFS cannot replace OS perfectly, but there is a correlation between PFS and OS under specific circumstances. Although the exact mechanism is unknown, PFS can represent OS in specific malignant tumors such as colorectal cancer and recurrent high-grade glioma27,28. Furthermore, one study emphasized that tumors with a shorter SPP (<12 months) had a relationship between PFS and OS compared to tumors with a longer SPP (≥12 months) (r=0.64 and r=0.38, respectively)29. In this study, the median SPP of the SCLC patients was 120 days (<12 months); therefore, our results support the association between PFS and OS.
In this study, PFS was not short in all patients with SCLC. Therefore, we propose that PFS reflects such tumor characteristics as growth rate or metastatic ability. For example, SCLC could be divided into rapid- and slow-growing types by PFS; this distinction may be related to molecular genetic factors that were not analyzed in this study. Currently, primary mutations in oncogenes and tumor suppressor genes are known to be molecular genetic factors in SCLC30. Primary oncogenes direct transcription, cell growth, and cell cycle progression. MYC oncogenes (CMYC, NMYC, and LMYC) are related to SCLC. The overactivation of MYC results in the rapid growth of cancer cells and differentiation failure, which are found in 40% of patients with disease progression after chemotherapy31,32. The tumor suppressor genes FHIT, RASSF1, retinoic acid receptor β, and FUS1 are particularly associated with SCLC, and mutation of these genes is directly or indirectly related to tumor growth and differentiation30. However, the type of genetic factor that is related to long-term survival in SCLC remains unknown; thus, additional studies are needed.
A potential conclusion drawn from this study is that PFS reflects tumor characteristics or survival and is useful for managing SCLC patients by clinicians. According to one study, the tumor volume doubling time in SCLC was shorter than that in NSCLC (39-64 days vs. 61-269 days)33. A poor SCLC prognosis is due to its rapid progression rate and vulnerability of metastasis to other organs. In addition, there are few anticancer agents against SCLC compared with NSCLC, and drug resistance occurs more easily in SCLC patients. Accordingly, after first-line therapy, sequential treatment should be focused on tumors in life-threatening organs or that concern QOL, including pain or nutritional intake. Sequential therapy begun just after the detection of slight increases in tumor size can result in the absence of the treatment arm at a critical period due to drug resistance, which occurs from previously used drugs. To overcome these limitations in managing SCLC patients, making treatment decisions while considering the tumor characteristics before progressing to second-line treatment would be helpful. Prognostic factors related to survival will contribute to the prevention of QOL deterioration due to cytotoxic chemotherapy and drug resistance due to unnecessary treatment.
This study has three major limitations. First, 21.7% of patients were lost to follow-up. Although we lost track of these patients, those who survived >2 years were counted as long-term survivors. However, in patients with <2 years of follow-up, we could not estimate the actual survival length and they were not classified as long-term survivors. The latter group was comprised of 39 patients (14.5% of all patients). Second, differences in staging might exist regarding whether PET-CT was performed. In patients diagnosed after 2007, PET-CT was used for initial staging because the equipment was available at Chungnam National University Hospital. However, before 2007, some patients had PET-CT performed at another hospital, and PET-CT was not performed at our hospital; thus, tumor staging might be over- or underestimated. Third, some parameters such as SIADH, NSE which are known as prognostic factors were not analyzed in the present study. Because urine sodium level which is essential for diagnosing SIADH and positivity of NSE were not measured in most of patients.
In addition to the many prognostic factors discussed in the Introduction, this study found a relationship between long-term survival and serum CRP levels, TNM staging, and PFS. Among these factors, PFS in particular reflects the character of malignant tumors and may be useful for determining whether sequential therapy is necessary. Studies of the potential relationship between molecular factors involved in SCLC and long-term survival are needed.
Notes
No potential conflict of interest relevant to this article was reported.