Healthcare-Associated Pneumonia among Hospitalized Patients: Is It Different from Community Acquired Pneumonia?
Article information
Abstract
Background
The increasing number of outpatients with multidrug-resistant (MDR) pathogens has led to a new category of pneumonia, termed healthcare-associated pneumonia (HCAP). We determined the differences in etiology and outcomes between patients with HCAP and those with community-acquired pneumonia (CAP) to clarify the risk factors for HCAP mortality.
Methods
A retrospective study comparing patients with HCAP and CAP at Jeju National University Hospital. The primary outcome was 30-day mortality.
Results
A total of 483 patients (208 patients HCAP, 275 patients with CAP) were evaluated. Patients with HCAP were older than those with CAP (median, 74 years; interquartile range [IQR], 65-81 vs. median, 69 years; IQR, 52-78; p<0.0001). Streptococcus pneumoniae was the major pathogen in both groups, and MDR pathogens were isolated more frequently from patients with HCAP than with CAP (18.8% vs. 4.9%, p<0.0001). Initial pneumonia severity was greater in patients with HCAP than with CAP. The total 30-day mortality rate was 9.9% and was higher in patients with HCAP based on univariate analysis (16.3% vs. 5.1%; odds ratio (OR), 3.64; 95% confidence interval (CI), 1.90-6.99; p<0.0001). After adjusting for age, sex, comorbidities, and initial severity, the association between HCAP and 30-day mortality became non-significant (OR, 1.98; 95% CI, 0.94-4.18; p=0.167).
Conclusion
HCAP was a common cause of hospital admissions and was associated with a high mortality rate. This increased mortality was related primarily to age and initial clinical vital signs, rather than combination antibiotic therapy or type of pneumonia.
Introduction
Pneumonia is typically classified as community-acquired pneumonia (CAP), hospital-acquired pneumonia (HAP), or ventilator-associated hospital-acquired pneumonia (VAP). However, over the past few decades, some patients presenting as outpatients with pneumonia have been infected with multidrug-resistant (MDR) pathogens such as Pseudomonas aeruginosa, methicillin-resistant Staphylococcus aureus, and extended-spectrum β-lactamase producing Enterobacteriaceae1. The potential involvement of these MDR pathogens has led to a new category of pneumonia, termed healthcare-associated pneumonia (HCAP). In 2005, the American Thoracic Society (ATS) and the Infectious Disease Society of America (IDSA) published the first guidelines for managing adults with HCAP2. In these guidelines, HCAP is included in the spectrum of HAP and VAP, and all patients with HCAP require therapy with dual antipseudomonal antibiotics plus linezolid or vancomycin for MDR pathogens.
Although the new pneumonia classification system has been helpful for determining empirical antibiotic strategies, it is not without its disadvantages. First, because of a lack of large-scale prospective studies and limited epidemiological data, the clinical outcomes of the 2005 ATS/IDSA guidelines are still under discussion. Not all MDR pathogens are associated with all risk factors. In addition, risk factors for an MDR pathogen infection do not preclude the development of pneumonia caused by the typical CAP pathogens3.
Materials and Methods
This was a retrospective observational study conducted at Jeju National University Hospital, a 500-bed teaching hospital in Jeju, Republic of Korea, between January 2010 and December 2011. All consecutive patients with pneumonia admitted to the hospital through the emergency or outpatient department were eligible. One of the investigators (G.M.S) reviewed all 1,754 patients hospitalized at the Department of Respiratory and Allergy during the 2-year period to identify appropriate patients. Patients with HAP, those on a home mechanical ventilator, patients referred after antibiotic administration, and patients with human immunodeficiency virus infection or organ transplantation were excluded. We considered that patients who fulfilled the HCAP criteria but developed pneumonia within 10 days after discharge as having developed HAP. We classified study patients into CAP and HCAP groups, and compared clinical characteristics, severity, pathogen distribution, and outcomes between the two groups. Our study protocol was approved by the Institutional Review Board of Jeju National University Hospital (IRB no. 2012/08002).
1. Definition of pneumonia
Patients with pneumonia were defined as those presenting with a new radiographic infiltrate and at least two of the following symptoms or signs: cough, sputum production, hemoptysis, dyspnea, fever, pleuritic chest pain, or signs consistent with pneumonia on physical examination4. HCAP and CAP were defined according to the ATS/IDSA guidelines2,5. The HCAP group included patients with any of the following: 1) hospitalization for ≥2 days during the preceding 90 days; 2) residence in a nursing home or long-term care facility; 3) recent intravenous antibiotic therapy, chemotherapy, or wound care within the past 30 days before entering the study; 4) long-term dialysis, including hemodialysis and peritoneal dialysis, within 30 days of entering the study. The diagnosis and type of pneumonia were verified twice from the medical records by one of the investigators (G.M.S. or M.K.).
2. Microbiological evaluation
Recommended initial microbiological investigations were sputum and blood cultures, Legionella and pneumococcal urine antigen testing, an antibody titer for Mycoplasma pneumoniae, and stains and cultures in selected patients. Specimens acceptable for positive culture results included sputum, tracheal aspirate, bronchoscopic bronchoalveolar lavage fluid, and blood. Blood culture results were accepted if the same microorganism was also identified in a respiratory specimen or if no other source for the positive blood culture was identified. Additionally, a positive Legionella urine antigen result, an antibody titer for an atypical pathogen that changed to 4-fold or converted to positive, and a positive polymerase chain reaction for Mycobacterium tuberculosis were considered to indicate etiological pathogens.
Antibiotic therapy was classified as being inappropriate if the initially prescribed antibiotics were not active against the identified pathogens, based on in vitro susceptibility testing. Exceptionally, atypical pathogens such as M. pneumoniae, Chlamydophila species, and Legionella species were considered fully susceptible to fluoroquinolones or macrolides, and M. tuberculosis was excluded from being judged appropriately, because it requires a special antibiotic regimen.
3. Severity evaluation and outcomes
All patients were assessed for risk at admission using the Pneumonia Severity Index (PSI) and CURB-65 scores6,7. Patients were followed for 30 days from the date of admission. We recorded the 30-day mortality and the need for admission to the intensive care unit, mechanical ventilation, and/or vasopressor support. Initial treatment failure was defined as death during initial treatment or a change from the initial antibiotic to another drug after 48 hours because of clinical instability (e.g., lack of response, worsening of fever, or requirement for mechanical ventilation or vasopressors).
4. Statistical analysis
All statistical analyses were performed using PASW version 17 (SPSS Inc., Chicago, IL, USA). A level of p<0.05 was considered to indicate statistical significance. The χ-squared test was used to compare categorical variables. Continuous variables were analyzed using Student's t-test or the Mann-Whitney U test. Multivariate logistic regression analysis was performed to identify independent risk factors associated with 30-day mortality, as measured by the adjusted odds ratio (AOR) with 95% confidence intervals (CIs). The 30-day mortality was the dependent variable, and all potential risk factors with p-values <0.2 in the univariate analysis were independent variables in the multivariate logistic regression model.
Results
1. Patients
A total of 483 patients were evaluated (208 patients with HCAP and 275 with CAP) (Figure 1). Most of these patients were admitted due to a recent admission (54.3%) or residence in a nursing home (48.1%) (including overlapping cases). Eighty-four patients (40.4%) had more than two risk factors. The demographic and baseline clinical characteristics of the patients with HCAP and CAP are presented in Table 1. Patients with HCAP were older and had a lower body mass index than patients with CAP. No gender differences were observed between the groups, but comorbidities such as cancer, congestive heart failure, cerebrovascular disease, and dementia occurred more frequently in patients with HCAP.
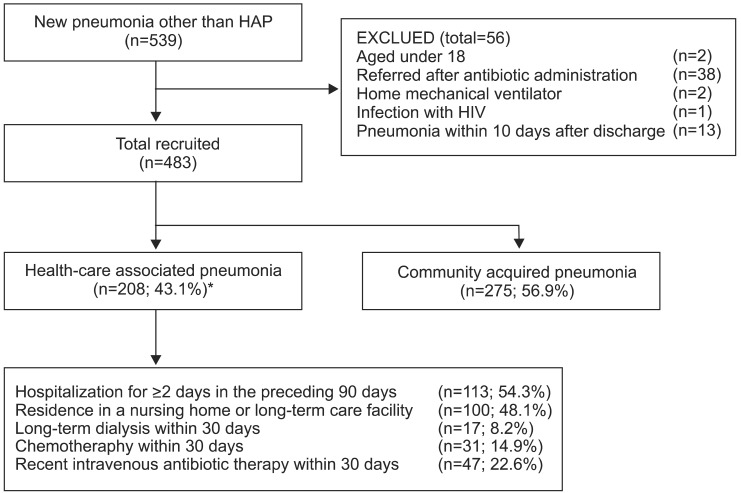
Flow diagram of the study population. *Including overlapping cases. HAP: hospital-acquired pneumonia; HIV: human immunodeficiency virus.
2. Microbiology and antibiotic treatments
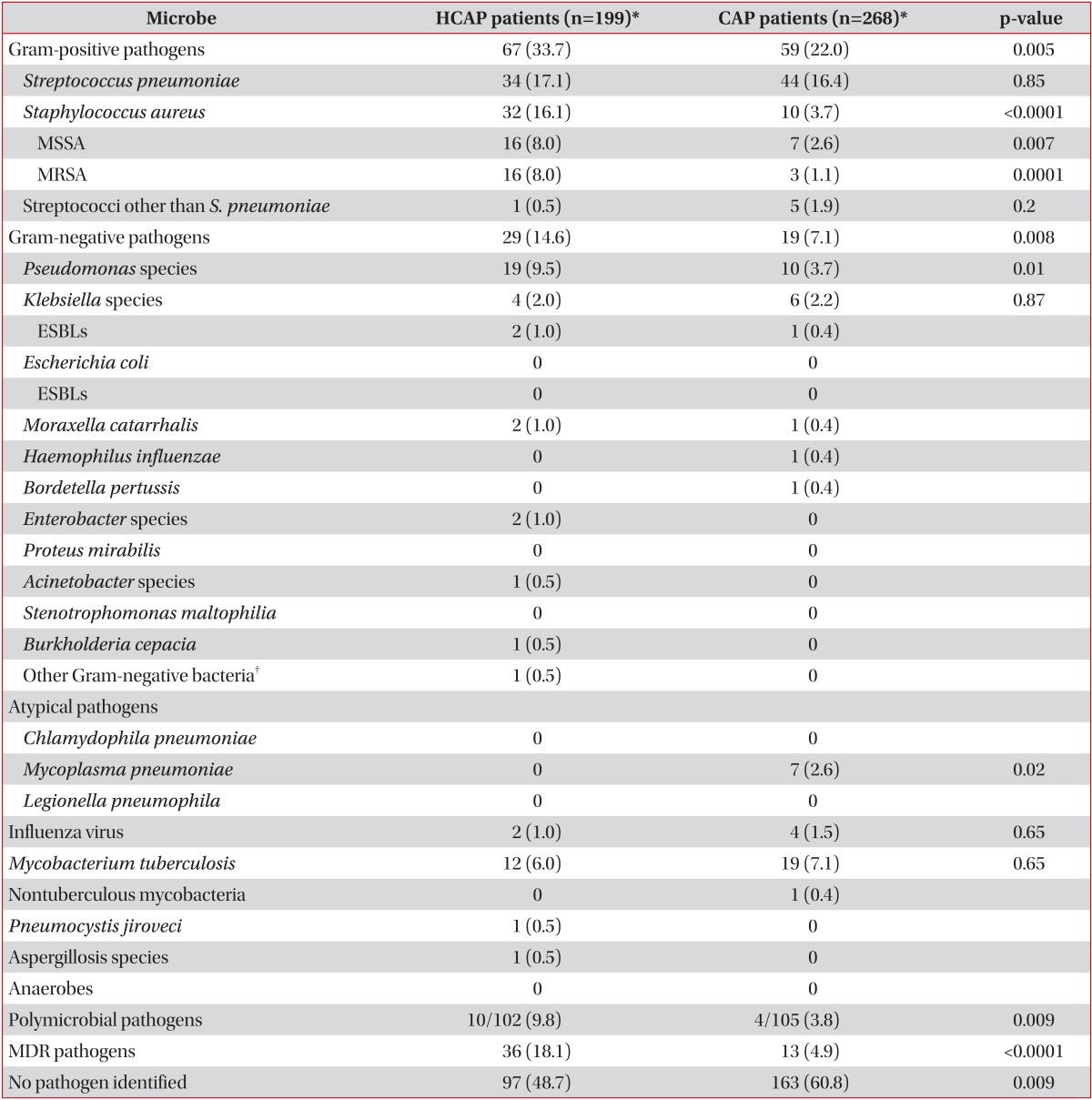
A positive microbiological diagnosis was made in 51.3% of the patients with HCAP compared with 39.2% of the patients with CAP (p=0.009). The frequencies of the organisms isolated in both groups are shown in Table 2. Streptococcus pneumoniae was the major pathogen in both groups, and S. aureus, P. aeruginosa, and polymicrobial pathogens were isolated more frequently from patients with HCAP than from those with CAP. M. tuberculosis was identified at an overall frequency of 6.4%, with no significant group difference (p=0.65).
After adjusting for age, comorbidities, and the HCAP risk group, the use of antibiotics within 90 days and tube feeding were significantly correlated with the occurrence of MDR pathogens (AOR, 8.03; 95% CI, 2.46-26.21; p=0.001 and AOR, 9.42; 95% CI, 3.40-26.13; p<0.0001, respectively).
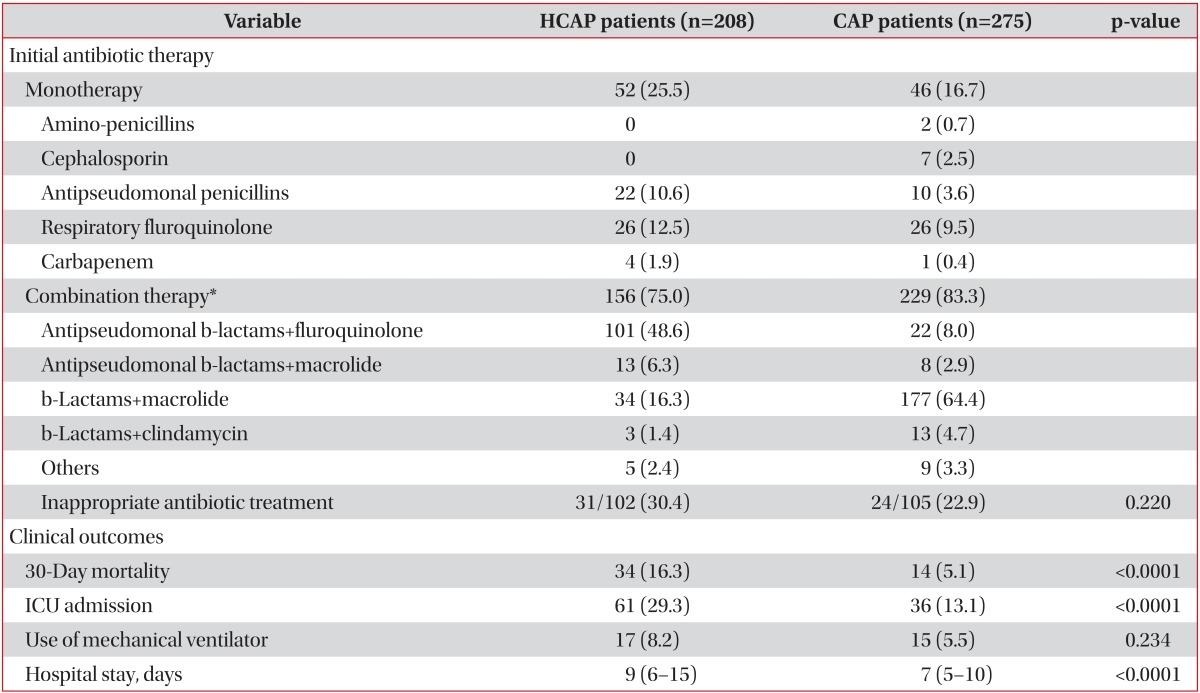
The most frequently prescribed initial antibiotic regimen was antipseudomonal β-lactam plus fluoroquinolone (48.6%; e.g., piperacillin/tazobactam 4.5 g q8hr plus levofloxacin 750 mg q24hr) for HCAP and third and fourth-generation cephalosporins and macrolides (64.4%; e.g., ceftriaxone 2 g q24hr plus azithromycin 500 mg q24hr) for CAP (data not shown). In addition, vancomycin was used in 15 patients with HCAP (7.2%) and eight patients with CAP (2.9%) (Table 3).
3. Severity of pneumonia and clinical outcomes
As shown Table 1, initial severities as measured by the PSI and CURB-65 were significantly higher in patients with HCAP than in those with CAP, and a probable case of aspiration pneumonia was more frequent in patients with HCAP.
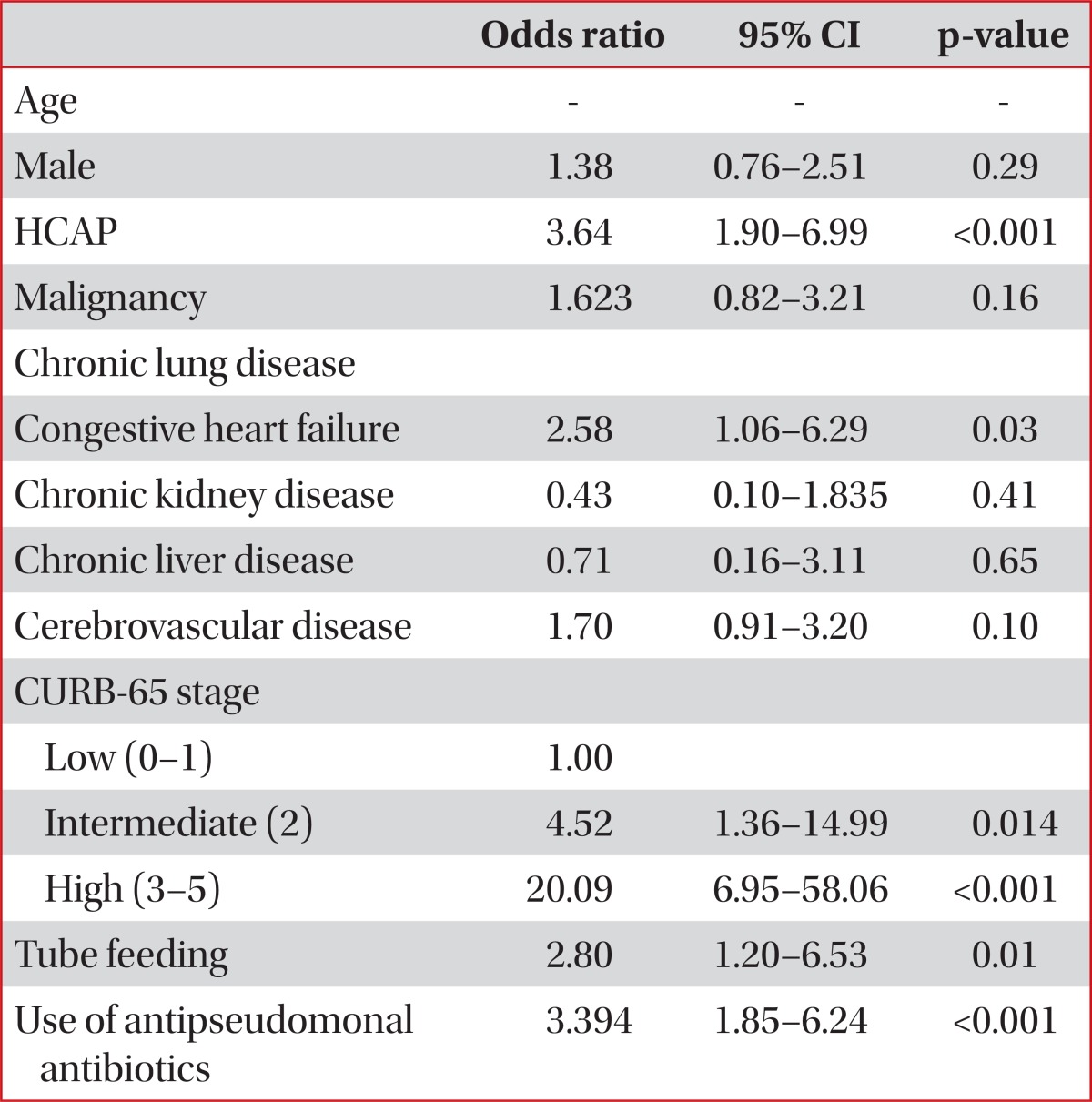
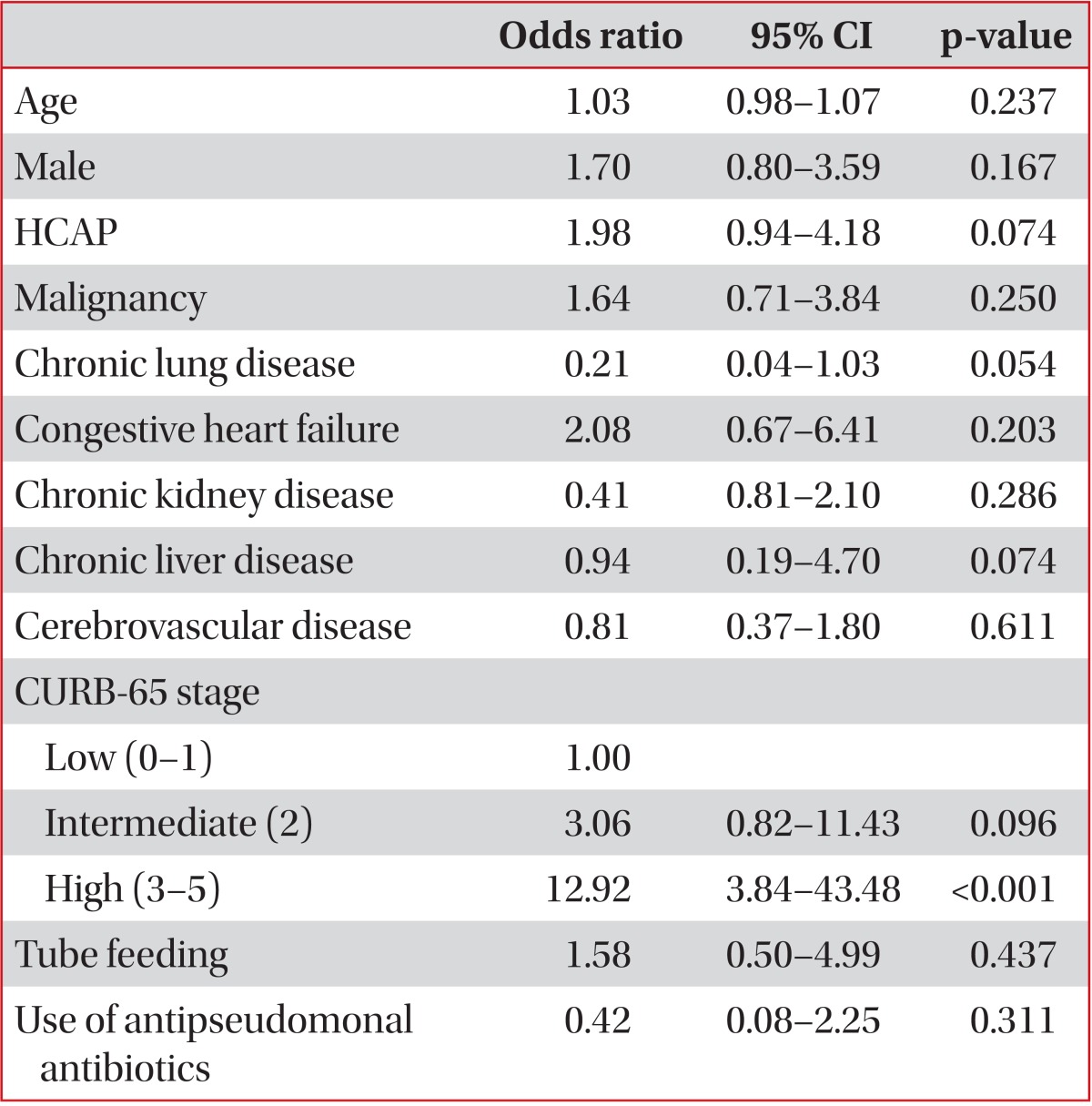
The overall 30-day mortality rate was 9.9%. In a univariate analysis, HCAP was associated with not only an increased 30-day mortality rate (HCAP, 16.3%; CAP, 5.1%; p<0.0001) but also an increased rate of mechanical ventilation or vasopressor support (HCAP, 20.7%; CAP, 9.5%; p<0.0001). The age distribution was one of the strongest risk factors for mortality. After excluding six unusually young cases (extreme values), the minimum age in the HCAP group was 42 years. Thus, we limited our study patients to those aged ≥42 years to reduce the effect of age. Forty-nine patients (6 with HCAP and 43 with CAP) were excluded, and there were no 30-day mortalities among these patients. In this limited study population, the univariate OR for HCAP and 30-day mortality was 3.15 (95% CI, 1.64-6.06; p<0.0001) (Table 4). However, after adjusting for age, sex, comorbidities, initial severity (CURB-65 score) and use of antipseudomonal antibiotics, the association between HCAP and the 30-day mortality rate became non-significant (OR, 1.98; 95% CI, 0.94-4.18; p=0.167) (Table 5). The Hosmer-Lemeshow test indicated a good fit for both models (p>0.05).
Inappropriate initial antimicrobial treatment was significantly higher in patients with HCAP than in those with CAP (p=0.047), but it did not correlate with 30-day mortality (p=0.293).
Among patients with HCAP, the use of antipseudomonal antibiotics as an initial therapy was not associated with a decrease in 30-day mortality in either the univariate (p=0.09) or multivariate analysis (AOR, 1.67; 95% CI, 0.72-3.90; p=0.24). Of the HCAP risk factors, only residence in a nursing home was independently associated with an increase in 30-day mortality (AOR, 4.46; 95% CI, 1.37-13.90; p=0.013).
Discussion
HCAP is a new category of pneumonia with potential involvement of MDR pathogens and is distinct from CAP. The 2005 ATS/IDSA guidelines concluded that most patients with HCAP are at risk for MDR pathogen infection. Thus, broad spectrum therapy with triple antibiotics is recommended for the patients with HCAP2. Despite these recommendations, the evidence specifically related to patients with HCAP is weak, and no randomized controlled trials have been conducted. Of the 294 references cited by the ATS/IDSA guidelines, only seven originally related to HCAP, and these were about nursing home-associated pneumonia8-10. Kollef et al.1 published a large retrospective HCAP data series in the United States, shortly after the guidelines were introduced. Among patients with HCAP, about 50% had methicillin-resistant Staphylococcus aureus (MRSA) or P. aeruginosa, and overall mortality was similar to that of patients with HAP (19.8% and 18.8%, respectively). These findings were consistent in several subsequent United States studies11-14. However, many disagreements have occurred among physicians, and recently reported studies from countries other than the United States have suggested that the frequency of MDR pathogens is more similar to that with CAP15-20.
Our study aimed to determine the differences in etiology and outcomes between patients with HCAP and those with CAP, and to clarify antibiotic strategies for HCAP in a tertiary teaching hospital in Jeju, Korea. HCAP accounted for 43.1% of hospitalized patients with pneumonia in the present study, and the incidence of HCAP was higher than that in previous studies (17.3-38.0%)1,15,21,22. The definition of HCAP was widely variable in previous studies, and some studies included only culture-positive patients. Our analysis included only hospitalized pneumonia cases and studies performed at a referral hospital. Because of the retrospective nature of this study, the true incidence of HCAP cannot be estimated. However, it is of greater importance that more than one-third of hospitalized pneumonia cases may have MDR pathogens.
In the present study, the overall 30-day mortality rate was 9.9%. As in previous studies, the mortality rate of patients with HCAP was three times that of patients with CAP. However, no relationship between HCAP and mortality was observed after adjustments for confounding variables. Investigating the underlying reasons for the increased mortality was complicated for several reasons. First, patients with HCAP were older and had more frequent comorbidities. As these two factors are powerful prognostic factors for pneumonia, other factors would be easily diluted in a retrospective study. In addition, it is uncertain whether MDR pathogens and inappropriate antibiotics were directly associated with higher mortality in patients with HCAP. Previous data on critically ill patients have shown that inappropriate antibiotic treatment is related to higher mortality23-25. However, outcomes and initial extended antibiotics are not simply defined in patients with HCAP. Rello et al.26 reported that pneumococcal HCAP results in excess mortality that is independent of bacterial susceptibility. These differences in outcomes were probably due to differences in age, comorbidities, and intensive care unit admission criteria, rather than therapeutic decisions. Recent studies from the United States27 and Canada28 have suggested that therapy consistent with the 2005 ATS/IDSA guidelines fails to decrease mortality and in some cases, tends to correlate with increased mortality.
About 40% of the patients in the HCAP group had more than two risk factors. It is unclear whether each healthcare environment had the same risk for MDR pathogens, or whether there are multiple risk factors for MDR pathogens. According to the current definition, HCAP is a distillation of multiple risk factors; thus, each patient must be considered individually, particularly with regard to performance status. For example, the risk for infection with MDR pathogens in a nursing home resident who has dementia but maintains daily self-care activities is different from the risk in a patient who is in a vegetative state with a tracheostomy and a feeding tube.
The ATS/IDSA guidelines for the empirical treatment of HCAP indicate that MRSA should be treated with either vancomycin or linezolid2,19. However, the impact of this recommendation on clinical outcomes, including mortality, is limited, and the prevalence of MRSA varies29,30. In the present study, MRSA was identified in 7.7% of the patients with HCAP, and 7.2% of the patients with HCAP were initially prescribed vancomycin. We could not analyze clinical outcomes and vancomycin use because the incidence of MRSA and frequency of vancomycin administration were too low.
M. tuberculosis was isolated from 5.8% of patients with HCAP and 6.9% of patients with CAP. In most previous HCAP studies, M. tuberculosis was not considered a major pathogen and was not evaluated. According to our data, M. tuberculosis is not rare in patients with HCAP, and acid fast bacilli staining and cultures should be considered in the initial diagnostic work-up in M. tuberculosis-endemic areas31,32.
Although study did not establish a clear guideline to choose antibiotics, it is important to know local epidemiologic data.
We found causative microorganisms in hospitalized pneumonia cases except those of HAP. Significantly more Pseudomonas species were identified in the HCAP group than in the CAP group, yet fewer than 10% of the Pseudomonas pneumonia cases occurred in the HCAP group. This suggests that the use of double or triple broad-spectrum antibiotics may not be justified, although we did not seek to establish clear guidelines for choosing antibiotics in the present study. After adjusting for potential confounders, the 30-day mortality rate was not significantly higher in the HCAP group compared with the CAP group. The observed higher mortality in patients with HCAP may be attributable mainly to the age of the patient and the initial clinical severity of the disease. While an unverified, broad definition of HCAP may obscure the importance of healthcare-associated MDR infections, it is nevertheless important to understand local epidemiological data on MDR infections. Unfortunately, few studies have evaluated HCAP epidemiology in Korea33,34.
Our study had some limitations that warrant consideration. First, because this was a retrospective study, unintentional data loss might have influenced the HCAP classification, despite our careful review of medications and hospitalization. We did not collect data on premorbid performance status or treatment limitations such as do not resuscitate orders. Therefore, we could not evaluate how performance status and treatment restrictions might have influenced clinical outcomes. Second, there are some limitations regarding available laboratory data. Haemophilus influenza is a major pathogen of adult pneumonia, but reported culture-positive results were extremely rare. No follow-up antibody titer tests were performed in any of the suspected M. pneumoniae patients. Third, consideration of special conditions such as tracheostomy or a feeding tube in situ was lacking.
In conclusion, HCAP was a common cause of hospital admissions and was associated with a high mortality rate. However, the higher mortality rate in patients with HCAP was not related to MDR pathogens. Thus, broad-spectrum antibiotics should be used with caution in these patients. The regional variations in the incidence and microbiology of HCAP emphasize the importance of understanding the local epidemiology. Furthermore, heterogeneous HCAP data may be confusing, and the HCAP concept should be carefully defined. A well-designed substantial study is imminent.