|
 |
| Tuberc Respir Dis > Volume 75(6); 2013 > Article |
|
Abstract
Endobronchial metastasis of leiomyosarcoma is rare, but it can cause life-threatening complications, such as massive hemoptysis, respiratory failure or even death. The development of new endoscopic modalities allows for effective endobronchial management. We report three patients with endobronchial metastases from advanced leiomyosarcomas which caused bronchial obstruction. The bronchoscopic examinations revealed masses obstructing the left main bronchus in all three patients. After removing the endobronchial tumor via interventional bronchoscopy, there was symptomatic and radiologic improvement. Moreover, the patients were able to undergo additional palliative chemotherapy. Therefore, endobronchial management of endobronchial tumors should be considered in the treatment of endobronchial metastasis, even in patients with advanced malignancies.
While pulmonary metastasis is common, endobronchial metastasis is rare in patients with leiomyosarcoma1. Nevertheless, it can cause dyspnea due to bronchial obstruction, hemoptysis, recurrent pulmonary infection, and ultimately respiratory failure or death. Giudice et al.2 reported a patient with bronchial metastasis of uterine leiomyosarcoma who developed progressive hemoptysis and died without proper management.
Bronchoscopy is an important therapeutic option for endobronchial metastasis, as well as a diagnostic modality. The evolution of interventional bronchoscopy enables debulking therapy for endobronchial tumors3. Mechanical debulking, electrocautery, diathermy, argon plasma coagulation, laser resection and cryoextraction can all provide immediate improvement, while brachytherapy and photodynamic therapy have delayed effects3,4.
Several studies have reported that the effect of interventional bronchoscopy for endobrochial metastases from malignant neoplasm, resolving endometrial metastases from colorectal cancer5, small cell lung cancer6, renal cell carcinoma7, anorectal melanoma8 and hypernephroma9. However, there is little information about interventional bronchoscopy for endobronchial metastasis from advanced leiomyosarcoma.
This paper reports three advanced leiomyosarcoma patients with successful amelioration of dyspnea using flexible bronchoscopy, which allowed them to undergo further palliative chemotherapy.
A 49-year-old female visited our hospital complaining of a painful mass in the right buttock and dyspnea. One month earlier, she had been diagnosed with leiomyosarcoma after excision of a mass in the right suprapubic area. Positron emission tomography-computed tomography (PET-CT) revealed metastases to the gluteal muscles and lungs.
Palliative chemotherapy was recommended after the surgical excision because of the severe hip pain. Meanwhile a chest radiograph revealed haziness of the left hemithorax suggesting atelectasis of the left lung (Figure 1A). Bronchoscopy disclosed a huge encapsulated mass involving the left main bronchus (Figure 2).
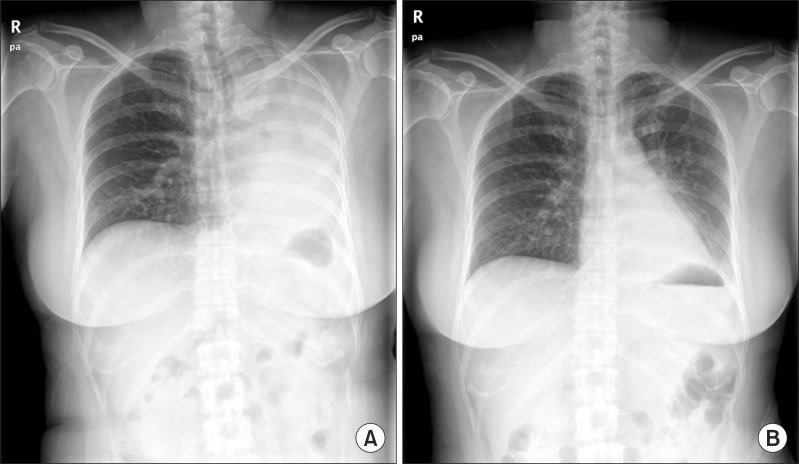
The tumor was removed bronchoscopically using a snare (Force FX TM-8C; Valley Lab, Boulder, CO, USA) and cryoextraction. A 5-cm encapsulated mass was extracted with a flexible cryoprobe (Erbokryo CA; ERBE Elektromedizin GmbH, T├╝bingen, Germany). After removing this huge mass, remnant tumor was seen in the left lower lobe. A chest radiograph after the bronchoscopic intervention showed improvement of the atelectasis (Figure 1B). Pathologic findings of endobronchial tumor showed a spindle cell neoplasm, which was consistent with metastatic leiomyosarcoma (Figure 3A, B).
After recanalization of the left main bronchus via interventional bronchoscopy, the dyspnea disappeared and the patient received additional chemotherapy. Nevertheless, the endobronchial metastasis progressed and endobronchial obstruction recurred at the same site 4 months later. Interventional bronchoscopy for debulking was repeated, which relieved her symptoms, except mild nonspecific chest discomfort. The patient did not want further chemotherapy because of progression of the underlying leiomyosarcoma. She discharged to palliative care.
A 40-year-old male was admitted the orthopedics department complaining of a mass on the right thigh. He underwent wide excision of this and pathology confirmed the diagnosis of leiomyosarcoma. Postoperative radiotherapy followed. Since multiple lung metastases were observed on PET-CT 1 month after radiotherapy, palliative chemotherapy followed. A craniotomy with tumor removal was performed for brain metastasis 1 year later.
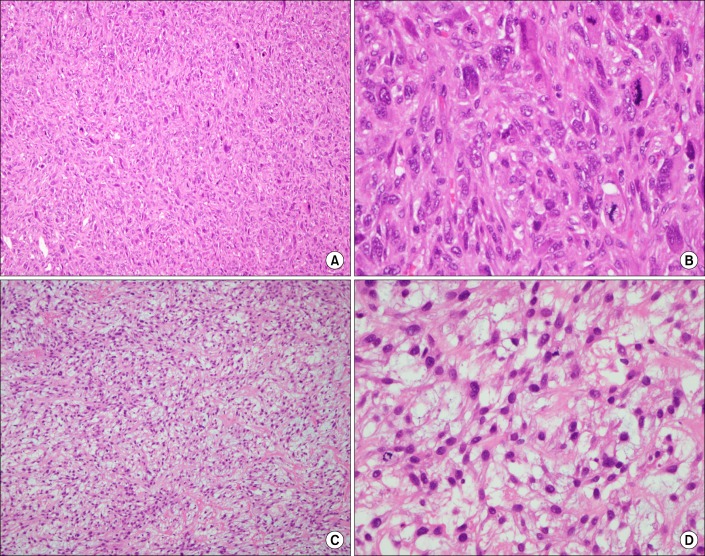
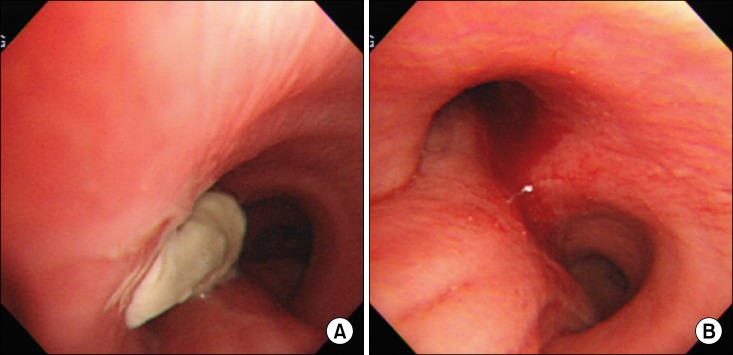
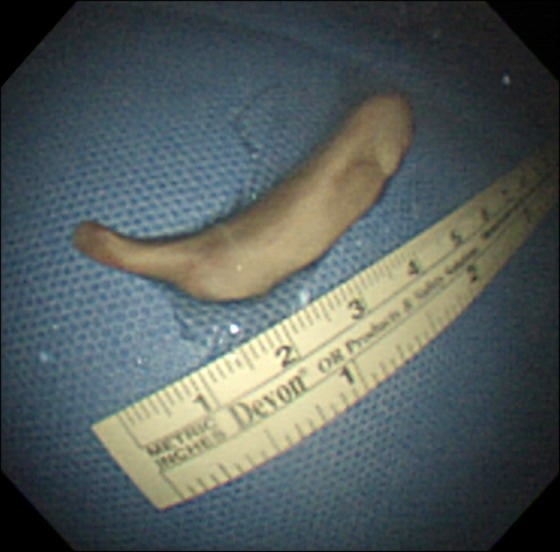
One month after the craniotomy, worsening dyspnea developed. Total collapse of the left lung was observed on chest radiography (Figure 4A). Bronchoscopy was performed and an endobronchial tumor was observed in the proximal left main bronchus (Figure 5). The tumor had a smooth mucosal surface and protruded into the left upper lobe bronchus. The tumor was removed using a snare and cryoextraction (Figure 6). This relieved the dyspnea and a chest radiograph showed improved aeration (Figure 4B). The pathologic specimen of bronchial tumor revealed metastatic leiomyosarcoma (Figure 3C, D). Subsequently, palliative chemotherapy with pazopanib was administered.
Five months after administering the pazopanib, the patient developed dyspnea and chest discomfort during expiration or position change. His cough worsened and produced blood-tinged sputum. Chest CT revealed on increased mass obstructing the left main bronchus again. Bronchoscopic examination showed endobronchial tumors obstructing the distal left main bronchus and openings of the left upper and lower lobes. Bronchoscopic tumor removal was repeated using cryoextraction. After debulking therapy, the symptoms were relieved and the patient was given additional palliative pazopanib chemotherapy.
A 38-year-old female visited our emergency room complaining of worsening dyspnea and blood-tinged sputum. She had undergone a total hysterectomy for a uterine mass 5 years earlier. Chest radiography revealed multiple masses of various sizes in both lungs, suggesting pulmonary metastases. Chest CT revealed an elongated bronchial lesion in the left main bronchus, almost obstructing it. Abdominal CT revealed an 8.8-cm enhancing soft tissue mass with adjacent bone destruction at the sacrum, suggesting bone metastasis. Further evaluation including a biopsy was performed, and leiomyosarcoma with metastasis to lung, bone, heart, and brain was diagnosed.
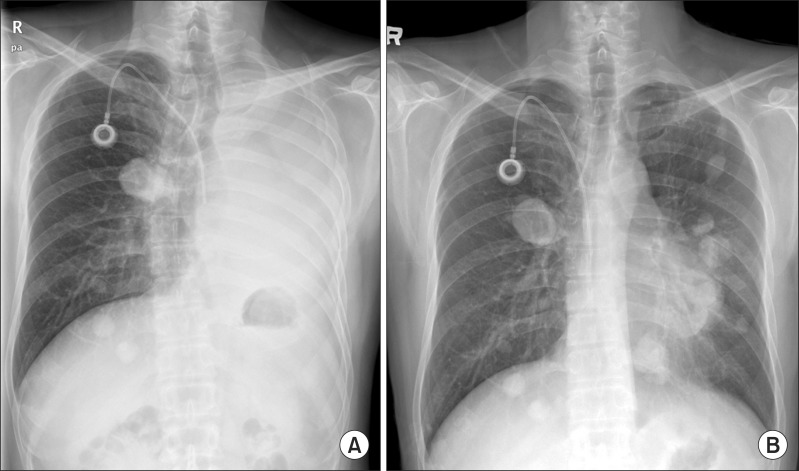
The dyspnea worsened despite a first cycle of chemotherapy. Chest radiography revealed increased opacity of the left hemithorax, suggesting atelectasis (Figure 7A). Bronchoscopy revealed an encapsulated pedunculated mobile mass in the proximal left main bronchus (Figure 8A). Recanalization of the left main bronchus was performed using a flexible bronchoscope with cryoextraction (Figure 8B). A 6-cm mass was extracted from the left main bronchus (Figure 9). The dyspnea was relieved and improvement of the atelectasis was observed on chest radiography (Figure 7B). Subsequently, the planned chemotherapy could be performed.
We describe three cases of bronchoscopic intervention using cryoextraction for endobronchial metastasis from leiomyosarcoma. Surgical resection was not indicated in any of the patients because of the advanced stage of their leiomyosarcomas. All had multiple metastases and two of them required repeated bronchoscopic intervention, which was effective and seemed safe. Bronchoscopic intervention both relieved symptoms and enabled further palliative treatment. All of our patients had an opportunity for further chemotherapy after bronchoscopic removal. The experiences with our three patients suggest that interventional bronchoscopy helps to alleviate respiratory symptoms and improves clinical status. It also enables the opportunity for further treatment.
The development of new endoscopic modalities in the last decade has facilitated endobronchial management with interventional bronchoscopy. Interventional bronchoscopy is a less-invasive modality for treating malignant airway obstruction. Cryorecanalization with a flexible cryoprobe is effective for treating endobronchial tumors. The reported success rate of cryoextraction is from 72.5%10 to 91.1%11. Recanalization of malignant endobronchial obstruction was successful in all three patients using cryoextraction.
Previous retrospective studies showed the effectiveness of interventional bronchoscopy in many types of neoplasm, including lung cancer, carcinoid, lymphoma, breast cancer, ovarian carcinoma, esophageal carcinoma, renal cell carcinoma, malignant melanoma, thyroid cancer, laryngopharyngeal carcinoma 10-12. Another showed that palliative treatment with interventional bronchoscopy to prevent asphyxia is a safe, effective method that can improve the quality of life in patients with endobronchial metastasis from colorectal cancer5.
There is little experience in using interventional bronchoscopy to treat endobronchial metastasis from leiomyosarcoma. Montero et al.13 reported two cases of pulmonary atelectasis due to a single metastasis to the main bronchus from a leiomyosarcoma of the uterus in one patients and leiomyosarcoma of the thigh in the other. Both patients underwent bronchoscopic intervention with photocoagulation before surgical resections. They suggested that interventional bronchoscopy could help to resolve local complications and alleviate symptoms when resection is not indicated.
To our knowledge, these are the first reported cases in which bronchoscopy was performed repeatedly for endobronchial metastasis in patients with advanced leiomyosarcoma, allowing further palliative chemotherapy. These cases suggest that interventional bronchoscopy improves the quality of life as well as provides an opportunity for further treatment, including chemotherapy, radiotherapy, or surgery. Bronchoscopic intervention could be a treatment option for endobronchial obstruction caused by a tumor and can be repeated if needed. Therefore, endoscopic management for endobronchial tumors should be considered in the treatment of endobronchial metastasis, even in patients with advanced malignancies.
References
1. Rose PG, Piver MS, Tsukada Y, Lau T. Patterns of metastasis in uterine sarcoma: an autopsy study. Cancer 1989;63:935-938. PMID: 2914299.


2. Giudice JC, Komansky H, Gordon R. Endobronchial metastasis of uterine leiomyosarcoma. Jama 1979;241:1684PMID: 430724.

3. Du Rand IA, Barber PV, Goldring J, Lewis RA, Mandal S, Munavvar M, et al. Summary of the British Thoracic Society guidelines for advanced diagnostic and therapeutic flexible bronchoscopy in adults. Thorax 2011;66:1014-1015. PMID: 22003155.


4. Hetzel J, Bockeler M. Endoscopic treatment for tumorous obstructions of the tracheobronchial system: a comparison of available techniques. Pneumologie 2012;66:408-415. PMID: 22628181.


5. Fournel C, Bertoletti L, Nguyen B, Vergnon JM. Endobronchial metastases from colorectal cancers: natural history and role of interventional bronchoscopy. Respiration 2009;77:63-69. PMID: 18812690.


6. Kiani A, Khosravi A, Moghaddam AS, Jabbari H, Fakhri M. Long-term survival of a small cell lung cancer patient with proper endobronchial management. Pneumologia 2012;61:245-248. PMID: 23424951.

7. Tomita M, Shiono Y, Sugaya S, Ikemoto I, Oishi Y. A case of lung collapse caused by endobronchial metastasis from renal cell carcinoma reinflated with laser and electrosurgical snaring. Hinyokika Kiyo 2002;48:459-462. PMID: 12229189.

8. Heyman BM, Chung MM, Lark AL, Shofer S. Endobronchial metastasis from primary anorectal melanoma. Am J Case Rep 2013;14:253-257. PMID: 23875060.



9. Themelin D, Duchatelet P, Boudaka W, Lamy V. Endoscopic resection of an endobronchial hypernephroma metastasis using a polypectomy snare. Eur Respir J 1990;3:732-733. PMID: 2379579.


10. Yilmaz A, Aktas Z, Alici IO, Caglar A, Sazak H, Ulus F. Cryorecanalization: keys to success. Surg Endosc 2012;26:2969-2974. PMID: 22609980.


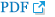
11. Schumann C, Hetzel M, Babiak AJ, Hetzel J, Merk T, Wibmer T, et al. Endobronchial tumor debulking with a flexible cryoprobe for immediate treatment of malignant stenosis. J Thorac Cardiovasc Surg 2010;139:997-1000. PMID: 19716140.


12. Hetzel M, Hetzel J, Schumann C, Marx N, Babiak A. Cryorecanalization: a new approach for the immediate management of acute airway obstruction. J Thorac Cardiovasc Surg 2004;127:1427-1431. PMID: 15116003.


13. Montero C, Valino P, Souto A, Fernandez Mdel M, Suarez J, Verea H. Endoscopic treatment of metastasis in the main bronchi from sarcoma: a report of 2 cases. Arch Bronconeumol 2010;46:40-43. PMID: 19540646.


Figure┬Ā1
(A) A chest radiograph revealed haziness of the left hemithorax, suggesting atelectasis of the left lung. (B) After bronchoscopic intervention, resolution of atelectasis was shown.
Figure┬Ā2
Bronchoscopic examination showed a huge encapsulated mass obstructing the distal left main bronchus.

Figure┬Ā3
Pathologic findings of endobronchial tumors revealed spindle cell neoplasm (A and C; H&E stain, ├Ś100). High power demonstrates spindle cells with blunt ended (cigar-shaped) nuclei and prominent cytologic atypia (B and D; H&E stain, ├Ś400).
Figure┬Ā4
(A) A chest radiograph revealed haziness of the left hemithorax, suggesting atelectasis of the left lung. (B) After bronchoscopic intervention, a chest radiograph showed improvement of atelectasis.
Figure┬Ā5
Bronchoscopic examination revealed a mass with smooth mucosal surface obstructing the proximal left main bronchus.

Figure┬Ā7
(A) A chest radiograph revealed haziness of the left hemithorax, suggesting atelectasis of the left lung. (B) After bronchoscopic intervention, chest radiography demonstrated resolution of atelectasis and multiple pulmonary metastases.

- TOOLS
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 5,539 View
- 21 Download
- Related articles
-
A case of multiple endobronchial hamartomas.1992 February;39(1)
A Case Report of Mediastinal Leiomyosarcoma.1994 December;41(6)
A Case of Primary Pulmonary Leiomyosarcoma.1997 April;44(2)
A Case of endobronchial metastasis from prostatic carcinoma.2000 October;49(4)
Diagnosis and Treatment of Endobronchial Actinomycosis.2005 June;58(6)



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Print
Print Download Citation
Download Citation