Pleural Fluid Pentraxin-3 for the Differential Diagnosis of Pleural Effusions
Article information
Abstract
Background
Conventional biomarkers cannot always establish the cause of pleural effusions; thus, alternative tests permitting rapid and accurate diagnosis are required. The primary aim of this study is to assess the ability of pentraxin-3 (PTX3) in order to diagnose the cause of pleural effusion and compare its efficacy to that of other previously identified biomarkers.
Methods
We studied 118 patients with pleural effusion, classified as transudates and exudates including malignant, tuberculous, and parapneumonic effusions (MPE, TPE, and PPE). The levels of PTX3, C-reactive protein (CRP), procalcitonin (PCT) and lactate in the pleural fluid were assessed.
Results
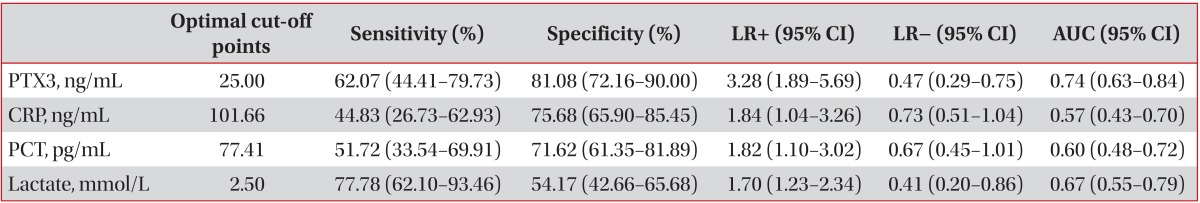
The levels of pleural fluid PTX3 were significantly higher in patients with PPE than in those with MPE or TPE. PTX3 yielded the most favorable discriminating ability to predict PPE from MPE or TPE by providing the following: area under the curve, 0.74 (95% confidence interval, 0.63-0.84), sensitivity, 62.07%; and specificity, 81.08% with a cut-off point of 25.00 ng/mL.
Conclusion
Our data suggests that PTX3 may allow improved differentiation of PPE from MPE or TPE compared to the previously identified biomarkers CRP and PCT.
Introduction
Pleural effusion is a common complication of many diseases, with an estimated 800,000 or more cases annually in the United States1. Parapneumonic (PPE), malignant (MPE), and tuberculous effusions (TPE) are the leading causes of exudative pleural effusion2. Although baseline studies including biochemical, microbiological, and cytological characteristics are helpful to differentiate the various causes of pleural effusion, the diagnostic confirmation is not always easily performed in clinical practice3,4. A variety of surrogate biomarkers have been proposed to facilitate differential diagnosis. Acute phase markers of inflammation, C-reactive protein (CRP) and procalcitonin (PCT) have recently been used to help differentiate infectious origin from other causes of pleural effusions2,5-8.
Pentraxin-3 (PTX3) is one of the long members of the pentraxin superfamily. PTX3 was originally identified as an interleukin-1 (IL-1)/tumor necrosis factor (TNF)-inducible gene9,10. Some evidences suggest that PTX3 is involved in both innate immunity and inflammatory responses, serving as an acute phase protein. PTX3 is produced at the site of infection and inflammation by tissue cells, macrophages, monocytes, and dendritic cells in response to injury, trauma, and infection10,11. Recently, PTX3 was reported as a potential candidate biomarker to differentiate PPE from other causes of pleural effusions12. To our knowledge, PTX3 has not been compared with other biomarkers to differentiate the etiology of pleural effusions.
The primary aim of this study was to assess the clinical utility of PTX3 whether it can differentiate PPE from other causes of pleural effusions. Moreover, the diagnostic performance of PTX3 was compared with previously identified biomarkers.
Materials and Methods
1. Patients
A total of 120 pleural fluid samples were collected prospectively from 120 consecutive patients who underwent diagnostic thoracentesis at the Division of Pulmonology of Seoul St. Mary's Hospital (Seoul, Korea) between September 2008 and February 2011. Two of these patients were excluded from this study because the causes of pleural effusions were indeterminate or other causes were plausible.
All subjects provided written informed consent. The study protocol was approved by the Institutional Review Board at St. Mary's Hospital, the Catholic University of Korea (Project approval number: KCMC08BR162).
2. Diagnostic criteria of pleural effusions
The determination of the etiology of pleural effusions was based on widely accepted criteria13. Briefly, characterization of exudates was validated using the criteria set out by Light et al14. as follows: pleural fluid protein/serum protein ratio of >0.5; pleural fluid lactate dehydrogenase (LDH)/serum LDH ratio of >0.6; and pleural fluid LDH level more than two-thirds of the upper limit of the normal serum value.
Effusions occurring secondary to lung cancer (diagnosed by the presence of malignant cells in cytological examination or biopsy specimens, or by histologically proven primary lung malignancy with the exclusion of any other causes of pleural effusions) and other malignancies (effusions that were clearly secondary to other malignancies, with the exclusion of other causes) were considered as MPE. TPE was diagnosed by the presence of a positive stain, polymerase chain reaction or Mycobacterium tuberculosis culture in the pleural fluid, sputum or pleural biopsy. TPE was also identified by the presence of typical caseating granulomas in pleural biopsy, or if exudative lymphocytic effusion with high adenosine deaminase (ADA) levels (>40 IU/L) cleared in response to antituberculous therapy. PPE was identified by the presence of pulmonary infections associated with acute febrile illness, pulmonary infiltrates, purulent sputum and a response to antibiotic treatment and/or pleural fluid neutrophilia. Other causes of pleural effusions were excluded.
3. Measurement of biomarkers
Pleural fluids were obtained from the first successful thoracentesis prior to treatment, taken immediately after each patient's admission. Samples were subsequently analyzed for total and differential cell count, gram stain, pH, glucose, protein, LDH, ADA and lactate. Additionally, cytological examination and cultures for common aerobic and anaerobic bacteria, and the acid-fast stain, polymerase chain reaction for M. tuberculosis, M. tuberculosis culture were routinely performed on all pleural samples. Aliquots of pleural fluid were immediately centrifuged at 1,500 ×g for 15 minutes at 4℃ and the supernatants stored at -80℃ for analysis.
Pleural fluid cytokines including PTX3 (detection limit, 0.025 ng/mL), CRP (detection limit, 0.010 ng/mL), and PCT (detection limit, 27 pg/mL) were measured using commercial enzyme-linked immunosorbent assay kits (PTX3, CRP: R&D Systems, Minneapolis, MN, USA and PCT: BioVendor, Karasek, Czech Republic) according to the manufacturer's instructions after all pleural fluid samples were obtained. The technician who measured these biomarkers was blinded to the clinical diagnosis. Pleural fluid pH and lactate measurements were analyzed using a blood gas analyzer (ABL800 FLEX; Radiometer, Copenhagen, Denmark) immediately after pleural fluid drainage.
4. Statistical analyses
Demographic data in patients with pleural effusions are presented as mean±SE or number (%). To compare parameters between exudates of different origins, we performed a one-way ANOVA followed by a post-hoc Duncan's test. To estimate the ability to discriminate among pleural effusions, a receiver operating characteristic analysis was performed and the area under the curve (AUC) was calculated.
All biomarkers were subject to transformation when they did not fit a Gaussian distribution. Thus, after logarithmic transformation, biomarkers were applied to evaluate the significance of each group and back-transformed for data presentation. We considered a two-sided p<0.05 to be statistically significant. All statistical analyses were performed using SAS version 9.2 (SAS Institute, Inc., Cary, NC, USA).
Results
1. Patient's demographics and baseline characteristics of pleural fluid
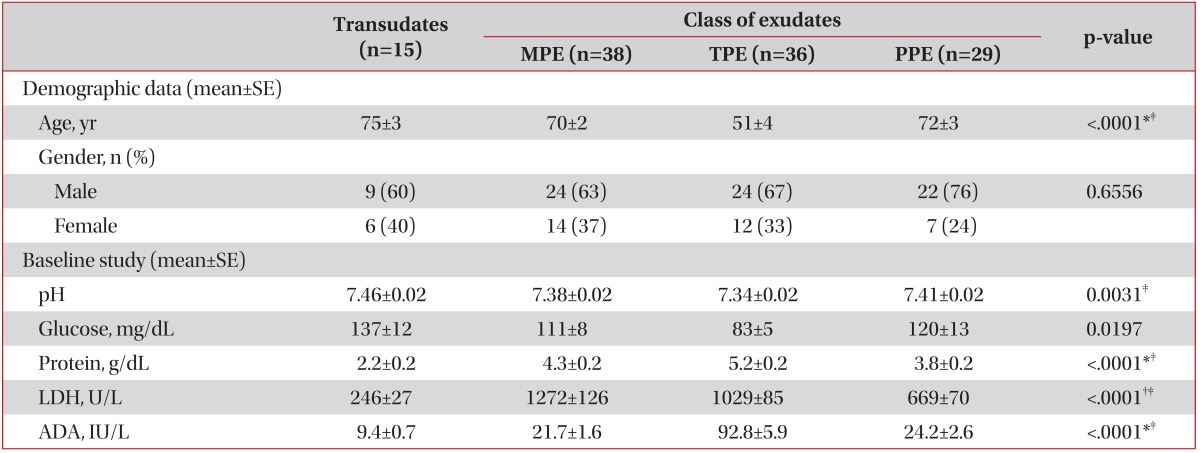
The study group included 79 males and 39 females with a mean age of 65.3 years. Of these, 15 (13%) had transudates, 38 (32%) had MPE, 36 (31%) had TPE, and 29 (25%) had PPE (Table 1).
Among 38 patients with MPE, 30 effusions occurred secondary to lung cancer (adenocarcinoma, 21; squamous cell carcinoma, 1; large cell carcinoma, 3; small cell carcinoma, 5), and 8 effusions occurred secondary to other malignancies (thyroid cancer, 3; breast cancer, 2; ovarian cancer, 1; endometrial cancer, 1; and stomach cancer, 1). Thirty-five out of 38 effusions were confirmed by cytological examination or biopsy specimens, and the others were clinically considered as MPE with the exclusion of other causes.
Patients with TPE were younger than those with other causes. Table 1 also lists the levels of pleural fluids pH, glucose, protein, LDH and ADA. Of the 36 patients with TPE, 23 cases were confirmed by the positive results of acid-fast stain, polymerase chain reaction for M. tuberculosis, or M. tuberculosis culture in the pleural fluid (n=12), or sputum or bronchoscopic studies (n=11). Other patients with TPE were diagnosed by exudative lymphocytic effusion with high ADA levels (>40 IU/L) that clinically cleared in response to anti-tuberculosis therapy.
2. Pleural fluid markers levels in exudates
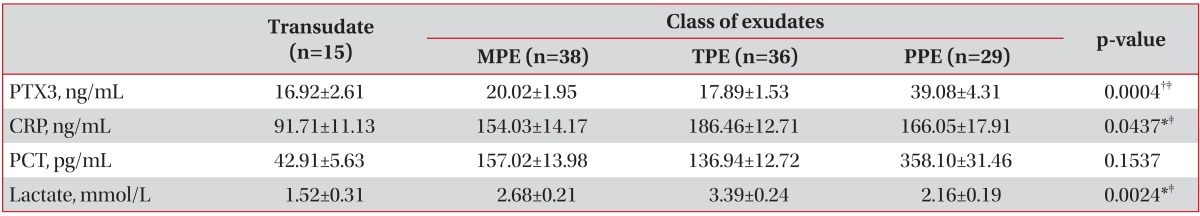
The mean levels of pleural fluid PTX3 in patients with PPE were significantly higher than in those with MPE or TPE (39.08 ng/mL vs. 20.02 ng/mL and 17.89 ng/mL, respectively; p<0.001) (Table 2, Figure 1). Table 3 compared the operating characteristics of various biomarkers for differentiating PPE from other exudates. PTX3 yielded the most favorable discriminating ability (AUC, 0.74; 95% confidence interval, 0.63-0.84; sensitivity, 62.07%; and specificity, 81.08%) to predict PPE from MPE or TPE, with a cut-off point of 25.00 ng/mL.

Levels of pleural fluid concentrations of PTX3 according to different etiologies of pleural effusions. MPE: malignant pleural effusion; TPE: tuberculous pleural effusion; PPE: parapneumonic pleural effusion; PTX3: pentraxin-3. *p<0.05.
Discussion
The purpose of this study was to assess the clinical utility of PTX3 for the differentiation of PPE from other causes of pleural effusions. Besides, the diagnostic performance of PTX3 was also evaluated with other surrogate biomarkers. We found that PTX3 has a more favorable diagnostic value for the differentiation of PPE from MPE and TPE, compared to previously reported biomarkers including CRP, PCT, and lactate.
Pentraxins are a superfamily of conserved proteins that includes short and long members10,15. Classic short pentraxins include CRP and serum amyloid P component, and PTX3 was the first long pentraxin to be discovered10. Although both short and long pentraxins share a pentraxin signature sequence of high homology and function as acute phase proteins involved in innate immunity and inflammation, they are encoded by different genes, each with different cellular origins under different regulatory mechanisms10.
The human CRP gene is located on chromosome 1q23, whilst PTX3 is located on 3q24-2810,15. In contrast to CRP, which is primarily produced in the liver under the stimulatory influence of IL-6 and TNF-α, PTX3 is produced by a variety of cells and tissues, notably dendritic cells and macrophages, in response to Toll-like receptor engagement and inflammatory cytokines such as TNF-α and IL-1β6,10,15-17. Both short pentraxins and PTX3 can recognize microbial structures and activate complements, and can act as a humoral amplification loop of the innate immune response15. However, PTX3 production is more rapidly inducible than CRP, so this distinct characteristic can distinguish between these factors9,15. As CRP is primarily produced in the liver and may arrive in the pleural space from plasma diffusion, higher levels of CRP in pleural fluids may simply reflect higher systemic levels6. This may imply that CRP is unlikely to reflect regional inflammation, but rather the extent of systemic inflammation6,15.
Several studies support PTX3 as a promising biomarker for acute inflammatory processes, as it is elevated during severe sepsis, septic shock, acute lung injury and acute respiratory distress syndrome17,18. The results of the present study were comparable with Ozsu's recent study, which reported that pleural PTX3 was positively correlated with pleural neutrophil percentage, and was increased much higher in PPE which was resulted from intense local inflammation than in MPE or in TPE12. They suggest PTX3 reflects local inflammation better than CRP. Furthermore, we demonstrated the diagnostic performance of PTX3 was superior to previous identified biomarkers, including CRP and PCT.
Pleural fluid CRP is a useful biomarker to the diagnosis and assessment of severity of PPE2,5,19. However, a number of studies investigating roles of CRP in pleural fluid were focused on the differentiation between complicated and uncomplicated PPEs8,19,20. In the present study, CRP levels in pleural fluid were also elevated in TPE and this finding is also noted in other previous reports6,21. It might be explained by profound tuberculous inflammation in TPE groups, represented by pH, glucose, protein and LDH. Levels of biomarkers, such as PTX3, CRP, PCT and lactate were higher in exudate than transudate, however, PCT levels were not significantly different among exudative pleural effusions. Therefore, novel biomarker adjuvant to conventional markers is clinically challenging, and pleural fluid PTX3 could be a promising biomarker for differentiating PPE from other exudates12.
Several limitations of this study should be acknowledged. First, the number of cases was relatively small. Second, we did not measure biomarker levels in the serum samples; pleural fluid/serum ratios have been reported as useful in the differentiation of the causes of pleural effusion6,7. Third, the diagnostic criteria of pleural effusions were not firmly strict, meaning a bias may have affected the differential diagnosis. We permitted histological, bacteriological, and clinical diagnostic categories to enroll patients with MPE, TPE and PPE, depending on the physician's judgment.
In conclusion, we demonstrated that PTX3 could be a novel and promising biomarker that can differentiate PPE from other causes of exudative pleural effusions. Moreover, PTX3 showed more favorable differentiating ability compared to previously reported biomarkers such as CRP and PCT. Further large scale studies are needed to elucidate the role of PTX3 in exudative pleural effusion.
Acknowledgements
This research was supported by the Seoul St. Mary's Clinical Medicine Research Program (2012) through the Catholic University of Korea. Statistical consultation was supported by the Catholic Research Coordinating Center of the Korea Health 21 R&D Project (A070001), Ministry of Health & Welfare, Republic of Korea.