 |
 |
| Tuberc Respir Dis > Volume 75(5); 2013 > Article |
|
Abstract
Background
We investigated whether prunetin significantly affects tumor necrosis factor-╬▒ (TNF-╬▒)-induced MUC5AC mucin gene expression, production, inhibitory kappa B (I╬║B) degradation and nuclear factor kappa B (NF-╬║B) p65 translocation in human airway epithelial cells.
Methods
Confluent NCI-H292 cells were pretreated with prunetin for 30 minutes and then stimulated with TNF-╬▒ for 24 hours or the indicated periods. MUC5AC mucin gene expression and mucin protein production were measured by reverse transcription polymerase chain reaction and enzyme-linked immunosorbent assay, respectively. The effect of prunetin on TNF-╬▒-induced degradation of I╬║B and translocation of NF-╬║B p65 was investigated by western blot analysis.
Mucus in the airway is very important in defense against invading pathogenic microorganisms, chemicals and particles. The protective function of airway mucus is due to the viscoelasticity of mucins. Mucins are macromolecular glycoproteins present in the airway mucus and have peptide backbones and carbohydrate branches. To date, twenty one or more MUC genes have been reported as coding the peptide backbone of human mucins and, among them, MUC5AC is remarkably expressed in airway goblet cells. However, any abnormality in the quality or quantity of mucins not only cause altered airway physiology but may also impair host defenses often leading to serious airway inflammatory diseases including chronic bronchitis, cystic fibrosis, asthma, and bronchiectasis1,2. Therefore, we suggest it is valuable to find the possible activity of controlling (inhibiting) the excessive mucin production (secretion) by the natural products derived from various medicinal plants that have been used for the management of airway diseases in folk medicine3-6. According to many reports, prunetin, a flavonoidal compound derived from licorice, Glycyrrhiza glabra L., showed inhibition of activities of phosphodiesterase, aldehyde dehydrogenase, alcohol dehydrogenase and antioxidative activities7-10. In our previous study, we demonstrated that prunetin inhibited epidermal growth factor (EGF)- or phorbol esters (PMA)-induced MUC5AC protein and gene expression in MUC5AC-producing NCI-H292 cells6. However, to the best of our knowledge, there are no reports about the effect of prunetin on mucin gene expression, production, degradation of inhibitory kappa B (I╬║B) and translocation of nuclear factor kappa B (NF-╬║B) p65 stimulated by tumor necrosis factor-╬▒ (TNF-╬▒) from airway epithelial cells. Therefore, in this study, we checked whether prunetin affects airway MUC5AC gene expression, production, degradation of I╬║B and translocation of NF-╬║B p65 induced by TNF-╬▒ from NCI-H292 cells, a human pulmonary mucoepidermoid cell line, which are frequently used for the purpose of elucidating intracellular signaling pathways involved in airway mucin production and gene expression11-13.
All the chemicals and reagents used in this study including prunetin (purity, 98.0%) were purchased from Sigma (St. Louis, MO, USA) unless otherwise specified. Anti-NF-╬║B p65, anti-I╬║B╬▒, anti-actin, and anti-poly(ADP-ribose) polymerase antibodies were purchased from Santacruz Biotechnology (Santa Cruz, CA, USA).
NCI-H292 cells, a human pulmonary mucoepidermoid carcinoma cell line, were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA) and cultured in RPMI 1640 supplemented with 10% fetal bovine serum (FBS) in the presence of penicillin (100 units/mL), streptomycin (100 ┬Ąg/mL) and HEPES (25 mM) at 37Ōäā in a humidified 5% CO2, 95% air water-jacketed incubator. For serum deprivation, at 60% confluence, cultures were washed twice with phosphate-buffered saline (PBS) and recultured in RPMI 1640 with 0.2% FBS for 24 hours.
After 24 hours of serum deprivation, cells were pretreated with varying concentrations of prunetin for 30 minutes and treated with TNF-╬▒ (10 ng/mL, 0.2 nM) for 24 hours in serum-free RPMI 1640. Prunetin was dissolved in dimethylsulfoxide and treated in culture medium (final concentrations of dimethylsulfoxide, 0.5%). 0.5% dimethylsulfoxide did not affect mucin gene expression and production from NCI-H292 cells. After 24 hours, cells were lysed with buffer solution containing 20 mM Tris, 0.5% NP-40, 250 mM NaCl, 3 mM EDTA, 3 mM EGTA and protease inhibitor cocktail (Roche Diagnostics, IN, USA) and collected to measure the production of MUC5AC protein (in 24-well culture plate). The total RNA was extracted for measuring the expression of MUC5AC gene (in 6-well culture plate) by using reverse transcription polymerase chain reaction (RT-PCR). For western blot analysis, cells were treated with prunetin for 24 hours and then treated with TNF-╬▒ for the indicated periods.
MUC5AC protein was measured by using ELISA. Cell lysates were prepared with PBS at 1:10 dilution, and 100 ┬ĄL of each sample was incubated at 42Ōäā in a 96-well plate, until being dry. Plates were washed three times with PBS and blocked with 2% bovine serum albumin for 1 hour at room temperature. Plates were again washed three times with PBS and then incubated with 100 ┬ĄL of 45M1, a mouse monoclonal MUC5AC antibody (1:200, NeoMarkers, Fremont, CA, USA) which was diluted with PBS containing 0.05% Tween 20 and dispensed into each well. After 1 hour, the wells were washed three times with PBS, and 100 ┬ĄL of horseradish peroxidase-goat anti-mouse IgG conjugate (1:3,000) was dispensed into each well. After 1 hour, plates were washed three times with PBS. Color reaction was developed with 3,3',5,5'-tetramethylbenzidine (TMB) peroxide solution and stopped with 1 N H2SO4. Absorbance was read at 450 nm.
Total RNA was isolated by using Easy-BLUE Extraction Kit (Intron Biotechnology Inc., Seongnam, Korea) and reverse transcribed by using AccuPower RT Premix (Bioneer Corp., Daejeon, Korea) according to the manufacturer's instructions. Two micrograms of total RNA was primed with 1 ┬Ąg of oligo(dT) in a final volume of 30 ┬ĄL (RT reaction). Two microliters of RT reaction product was PCR amplified in a 20 ┬ĄL by using Thermoprime Plus DNA Polymerase (ABgene, Rochester, NY, USA). Primers for MUC5AC were (forward) 5'-TGA TCA TCC AGC AGG GCT-3' and (reverse) 5'-CCG AGC TCA GAG GAC ATA TGG G-3'. As quantitative controls, primers for Rig/S15 rRNA, which encodes a small ribosomal subunit protein, a housekeeping gene that was constitutively expressed, were used. Primers for Rig/S15 were (forward) 5'-TTC CGC AAG TTC ACC TAC C-3' and (reverse) 5'-CGG GCC GGC CAT GCT TTA CG-3'. The PCR mixture was denatured at 94Ōäā for 5 minutes followed by 35 cycles at 94Ōäā for 30 seconds, 60Ōäā for 30 seconds and 72Ōäā for 30 seconds and for final extension, 1 cycle at 72Ōäā for 10 minutes. After PCR, 15 ┬ĄL of PCR products were subjected to 1% agarose gel electrophoresis and visualized with ethidium bromide under a transilluminator.
NCI-H292 cells (confluent in 150 mm culture dish) were pretreated for 24 hours at 37Ōäā with 50 ┬ĄM of prunetin and then stimulated with TNF-╬▒ (50 ng/mL, 1.0 nM) for the indicated periods. After the treatment, the cells were harvested using 3├Ś trypsin-EDTA solution and then centrifuged in a microcentrifuge (1,200 rpm, 3 minutes, 4Ōäā). The supernatant was discarded and the cell pellet was washed by suspending in PBS. The cytoplamic and nuclear protein fraction were extracted using NE-PER nuclear and cytoplasmic extraction reagent (Thermo-Pierce Scientific, Waltham, MA, USA) according to the manufacturer's instructions. Both extracts were stored at -20Ōäā. Protein content in extract was determined by Bradford method.
Cytosolic, nuclear and whole cell extracts containing proteins (each 20-60 ┬Ąg as protein) were subjected to 7-15% sodium dodecyl sulfate polyacrylamide gel electrophoresis and then transferred onto the polyvinylidene difluoride membrane. The blots were blocked using 5% skim milk and probed with appropriate primary antibody in blocking buffer overnight at 4Ōäā. The membrane was washed with PBS and then probed with the secondary antibody conjugated with horseradish peroxidase (Calbiochem, La Jolla, CA, USA). Immunoreactive bands were detected by an enhanced chemiluminescence kit (Pierce ECL western blotting substrate; Thermo Scientific).
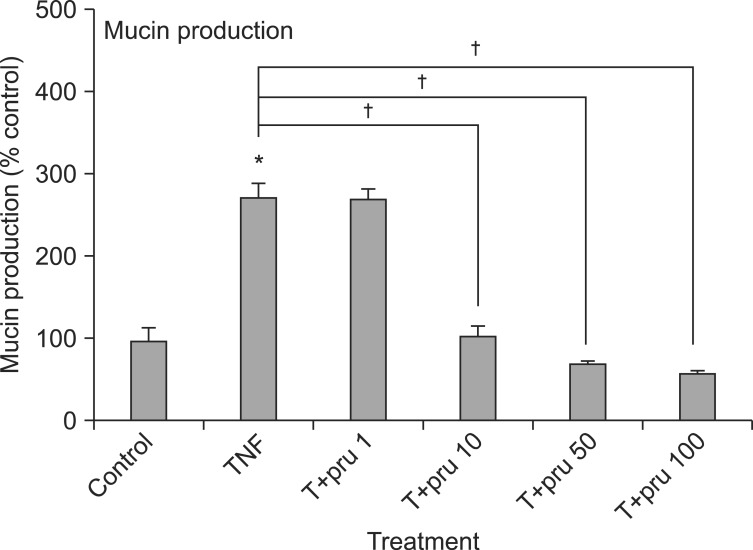
Prunetin inhibited TNF-╬▒-induced MUC5AC mucin production. The amounts of MUC5AC mucin in the cells of prunetin-treated cultures were 100┬▒14%, 275┬▒15%, 269┬▒13%, 105┬▒12%, 71┬▒4%, and 59┬▒3% for control, TNF-╬▒ 10 ng/mL only, TNF-╬▒ plus prunetin 1 ┬ĄM, TNF-╬▒ plus prunetin 10 ┬ĄM, TNF-╬▒ plus prunetin 50 ┬ĄM and TNF-╬▒ plus prunetin 100 ┬ĄM, respectively (Figure 1).
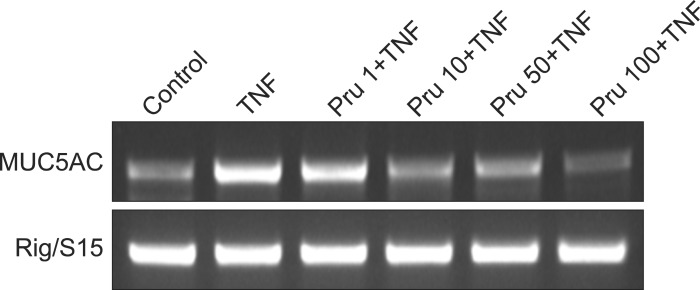
MUC5AC gene expression induced by TNF-╬▒ was also inhibited by pretreatment with 10, 50, and 100 ┬ĄM of prunetin (Figure 2).
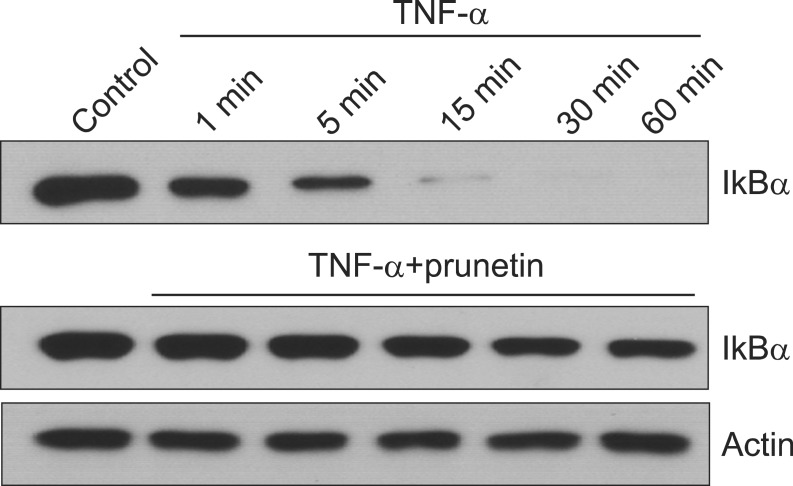
At 5 minutes after treatment, TNF-╬▒ showed the maximal induction of I╬║B╬▒ degradation. However, preincubation of NCI-H292 cells with 50 ┬ĄM of prunetin prior to TNF-╬▒ exposure suppressed I╬║B╬▒ degradation (Figure 3).
Nuclear translocation of NF-╬║B p65 by TNF-╬▒ was inhibited by pretreatment with 50 ┬ĄM of prunetin. In the nuclear fraction of the TNF-╬▒ only-treated cells, there was an increase in nuclear translocation of p65 gradually and reached optimal level at 30 minutes. However, in the cells treated with prunetin plus TNF-╬▒, the level of p65 was gradually decreased as compared to the TNF-╬▒ only-treated cells (Figure 4).
Trial to develop a useful pharmacological tool for regulating production and/or secretion of mucin, the macromolecular glycoproteins present in the airway mucus, is a promising approach to the effective control of severe inflammatory pulmonary diseases involving the hyperproduction and/or hypersecretion of airway mucus14. As aforementioned in introduction, prunetin, a flavonoidal compound derived from licorice, Glycyrrhiza glabra L., showed inhibition of activities of phosphodiesterase, aldehyde dehydrogenase, alcohol dehydrogenase and antioxidative activities7-10 and showed inhibition of EGF- or PMA-induced MUC5AC protein and gene expression in MUC5AC-producing NCI-H292 cells6. However, to the best of our knowledge, there are no reports about the effect of prunetin on mucin gene expression, production, degradation of I╬║B and translocation of NF-╬║B p65 stimulated by TNF-╬▒ in airway epithelial cells. As can be seen in results, we found that prunetin inhibited MUC5AC mucin gene expression and production of MUC5AC mucin protein, induced by TNF-╬▒ (Figures 1, 2). This result suggests that prunetin can regulate mucin gene expression and production induced by TNF-╬▒, through directly acting on airway epithelial cells. TNF-╬▒ has been reported to be a stimulant for secretion and gene expression of MUC5AC mucin in normal human airway epithelial cells13,15,16. After binding its receptor, TNF-╬▒ activates several intracellular signal transduction cascades among which the NF-╬║B pathway is very important. The transcription factor NF-╬║B has been a potential therapeutic target. NF-╬║B is a heterodimer composed of p65, p50, and I╬║B╬▒ subunits present in the cytoplasm as an inactive state. In response to various stimuli, the I╬║B╬▒ subunit is phosphorylated and degraded, thereby facilitating the translocation of p50-p65 heterodimer to the nucleus. p50-p65 acts as a transcription factor regulating the expression of numerous genes including MUC5AC17. As shown in results, prunetin affected the degradation of I╬║B╬▒ which is required for NF-╬║B activation of transcription. At 5 minutes after treatment, TNF-╬▒ showed the maximal induction of I╬║B╬▒ degradation. However, preincubation of NCI-H292 cells with prunetin prior to TNF-╬▒ exposure inhibited the degradation of I╬║B╬▒ (Figure 3). Also, nuclear translocation of NF-╬║B p65 by TNF╬▒ was inhibited by pretreatment with 50 ┬ĄM of prunetin. In the nuclear fraction of the TNF-╬▒ only-treated cells, there was an increase in nuclear translocation of p65 gradually and reached optimal level at 30 minutes. However, in the cells treated with prunetin plus TNF-╬▒, the level of p65 was gradually decreased as compared to the TNF-╬▒ only-treated cells (Figure 4). The result from this study suggests a possibility of using prunetin as a new efficacious mucoregulator for pulmonary diseases especially in conjunction with inflammation, although further studies are essential. Taken together, the inhibitory actions of prunetin on airway mucin gene expression and production might explain, at least in part, the traditional use of Glycyrrhiza glabra L., as an anti-inflammatory and anti-allergic agent for airway inflammatory diseases, in traditional oriental medicine. We suggest it is valuable both to find the natural products that have specific inhibitory effects on mucin gene expression and/or production and to search the optimal chemical moieties derived from the chemical structure of prunetin which can be useful as an efficacious regulator for mucin production in hypersecretory status of various chronic pulmonary diseases, through future studies.
Acknowledgements
This research was supported by a grant (12172KFDA989) from Korea Food & Drug Administration in 2012.
References
1. Adler KB, Li Y. Airway epithelium and mucus: intracellular signaling pathways for gene expression and secretion. Am J Respir Cell Mol Biol 2001;25:397-400. PMID: 11694442.


2. Basbaum C, Lemjabbar H, Longphre M, Li D, Gensch E, McNamara N. Control of mucin transcription by diverse injury-induced signaling pathways. Am J Respir Crit Care Med 1999;160(5 Pt 2):S44-S48. PMID: 10556169.


3. Heo HJ, Lee SY, Lee MN, Lee HJ, Seok JH, Lee CJ. Genistein and curcumin suppress epidermal growth factor-induced MUC5AC mucin production and gene expression from human airway epithelial cells. Phytother Res 2009;23:1458-1461. PMID: 19288529.


4. Lee HJ, Lee SY, Bae HS, Kim JH, Chang GT, Seok JH, et al. Inhibition of airway MUC5AC mucin production and gene expression induced by epidermal growth factor or phorbol ester by glycyrrhizin and carbenoxolone. Phytomedicine 2011;18:743-747. PMID: 21146382.


5. Lee HJ, Lee SY, Jeon BK, Lee JW, Kim YS, Lee MN, et al. Effect of platycodin D on airway MUC5AC mucin production and gene expression induced by growth factor and proinflammatory factor. Biomol Ther 2010;18:294-299.


6. Lee HJ, Lee SY, Lee MN, Kim JH, Chang GT, Seok JH, et al. Inhibition of secretion, production and gene expression of mucin from cultured airway epithelial cells by prunetin. Phytother Res 2011;25:1196-1200. PMID: 21305630.


7. Jung HA, Kim AR, Chung HY, Choi JS. In vitro antioxidant activity of some selected Prunus species in Korea. Arch Pharm Res 2002;25:865-872. PMID: 12510840.



8. Keung WM. Biochemical studies of a new class of alcohol dehydrogenase inhibitors from Radix puerariae. Alcohol Clin Exp Res 1993;17:1254-1260. PMID: 8116840.


9. Ko WC, Shih CM, Lai YH, Chen JH, Huang HL. Inhibitory effects of flavonoids on phosphodiesterase isozymes from guinea pig and their structure-activity relationships. Biochem Pharmacol 2004;68:2087-2094. PMID: 15476679.


10. Sheikh S, Weiner H. Allosteric inhibition of human liver aldehyde dehydrogenase by the isoflavone prunetin. Biochem Pharmacol 1997;53:471-478. PMID: 9105397.


11. Li JD, Dohrman AF, Gallup M, Miyata S, Gum JR, Kim YS, et al. Transcriptional activation of mucin by Pseudomonas aeruginosa lipopolysaccharide in the pathogenesis of cystic fibrosis lung disease. Proc Natl Acad Sci U S A 1997;94:967-972. PMID: 9023366.



12. Takeyama K, Dabbagh K, Lee HM, Agusti C, Lausier JA, Ueki IF, et al. Epidermal growth factor system regulates mucin production in airways. Proc Natl Acad Sci U S A 1999;96:3081-3086. PMID: 10077640.



13. Shao MX, Ueki IF, Nadel JA. Tumor necrosis factor alpha-converting enzyme mediates MUC5AC mucin expression in cultured human airway epithelial cells. Proc Natl Acad Sci U S A 2003;100:11618-11623. PMID: 12972643.



14. Rogers DF, Barnes PJ. Treatment of airway mucus hypersecretion. Ann Med 2006;38:116-125. PMID: 16581697.


15. Song KS, Lee WJ, Chung KC, Koo JS, Yang EJ, Choi JY, et al. Interleukin-1 beta and tumor necrosis factor-alpha induce MUC5AC overexpression through a mechanism involving ERK/p38 mitogen-activated protein kinases-MSK1-CREB activation in human airway epithelial cells. J Biol Chem 2003;278:23243-23250. PMID: 12690113.


16. Fischer BM, Rochelle LG, Voynow JA, Akley NJ, Adler KB. Tumor necrosis factor-alpha stimulates mucin secretion and cyclic GMP production by guinea pig tracheal epithelial cells in vitro. Am J Respir Cell Mol Biol 1999;20:413-422. PMID: 10030839.


17. Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol 2002;2:725-734. PMID: 12360211.



Figure┬Ā1
Effect of prunetin on tumor necrosis factor-╬▒ (TNF-╬▒)-induced MUC5AC mucin production. NCI-H292 cells were pretreated with various concentrations of prunetin (1, 10, 50, and 100 ┬ĄM) for 30 minutes and then stimulated with TNF-╬▒ (10 ng/mL) for 24 hours. Cell lysates were collected for measurement of MUC5AC mucin production by enzyme-linked immunosorbent assay. Three independent experiments were performed and the representative data were shown. Each bar represents the mean┬▒SEM of three culture wells in comparison with that of the control, set at 100%. *Significantly different from control (p<0.05). ŌĆĀSignificantly different from TNF-╬▒ alone (p<0.05). Concentration unit is ┬ĄM. Pru: prunetin.
Figure┬Ā2
Effect of prunetin on tumor necrosis factor-╬▒ (TNF-╬▒)-induced MUC5AC mucin gene expression. NCI-H292 cells were pretreated with 1, 10, 50, and 100 ┬ĄM of prunetin for 30 minutes and then stimulated with TNF-╬▒ (10 ng/mL) for 24 hours, respectively. MUC5AC mucin gene expression was measured by reverse transcription polymerase chain reaction. As a quantitative control, Rig/S15 rRNA, which encodes a small ribosomal subunit protein, a housekeeping gene that was constitutively expressed, was used. Three independent experiments were performed and the representative data were shown. Concentration unit is ┬ĄM. Pru: prunetin.
Figure┬Ā3
Effect of prunetin on tumor necrosis factor-╬▒ (TNF-╬▒)-induced degradation of inhibitory kappa B alpha (I╬║B╬▒). NCI-H292 cells were incubated with 50 ┬ĄM prunetin for 24 hours and treated with TNF-╬▒ (50 ng/mL) for the indicated periods. Cytoplasmic extracts were prepared and analyzed by western blot using the antibody against anti-I╬║B╬▒. The results shown are representative of three independent experiments. Equal protein loading was evaluated by ╬▓-actin levels.
Figure┬Ā4
Effect of prunetin on tumor necrosis factor-╬▒ (TNF-╬▒)-induced nuclear translocation of nuclear factor kappa B (NF-╬║B) p65. NCI-H292 cells were either untreated or pretreated with 50 ┬ĄM prunetin for 24 hours at 37Ōäā and then stimulated with TNF-╬▒ (50 ng/mL) for the indicated periods. Nuclear protein extracts were prepared and resolved on 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis, transferred onto a polyvinylidene difluoride membrane, and probed with an antibody against p65. The results shown are the representative of three independent experiments. To ensure equal protein loading, the membrane was re-probed with the anti-poly(ADP-ribose) polymerase (PARP) antibody.



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Print
Print Download Citation
Download Citation




