Embolization of Multiple Systemic Artery to Pulmonary Artery Fistula with Recurrent Hemoptysis
Article information
Abstract
Herein, we report a case of multiple systemic arteries to pulmonary artery fistulas without any underlying causes, presenting recurrent hemoptysis. Transcatheter embolization was successfully performed several times on multiple systemic feeding arteries. Multiple systemic arteries to pulmonary fistulas can be a source of uncontrolled bleeding, and embolization may be a reasonable therapeutic option to control the bleeding.
Introduction
Systemic artery to pulmonary artery fistulas (SA-PAFs) are known to develop in patients with congenital heart disease, which is combined with right ventricular outflow or pulmonary artery obstruction. In addition, they have been reported in cases of pulmonary infections, malignancy, trauma or surgery1-4. However, SA-PAFs in the general population without these factors are rare.
Herein, we describe a case of multiple SA-PAFs, which were the major cause of recurrent hemoptysis, in a patient without underlying disease. To control the hemoptysis, transcatheter embolization of the multiple feeding arteries of SA-PAFs was performed.
Case Report
A 51-year-old man was admitted to our hospital with recently developed hemoptysis. He was previously healthy and did not have any family history of hemoptysis. He had recurrent hemoptysis for three days and the amount was about 200 mL/day, but no cough, sputum, dyspnea, nor fever. The patient had a history of smoking with more than 30 pack-years. Upon examination, there were no crackles or wheezing suggesting airway diseases, and no cardiac murmurs. Laboratory data showed normal range of hemoglobin (15.8 g/dL), platelet (244,000/mm3), C-reactive protein (1.0 mg/dL), prothrombin international normalized ratio (1.0).
A chest radiograph revealed increased left parahilar opacity. For further evaluation, he underwent thin-section computed tomography (CT) scan (1.3 mm thickness). The arterial phase CT scan revealed about a 2.5 cm-sized well-enhanced elongated vascular mass abutting on the pericardium in the left upper lobe and an abnormally prominent peripheral pulmonary artery accompanying early enhancement of the left main pulmonary artery (Figure 1A). When the arteries around the lesion were traced, these originated from the mediastinal branch of the left internal mammary artery and the proximal portion of the left common carotid artery. On the lung window setting images, ground glass opacity suggesting aspirated blood was noticed around the lesion (Figure 1B). Based on the CT finding that the lesion had an arterial enhanced vascular mass with early enhancement of the pulmonary artery, this lesion was suggestive of a systemic artery to pulmonary artery fistula.
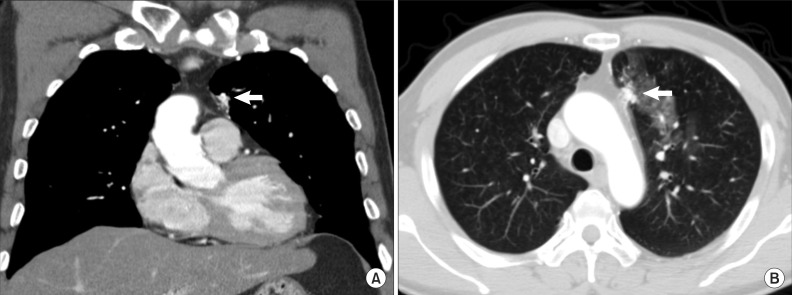
A chest computed tomography image in the mediastinal setting (A) shows that a vascular structure (arrow) abutting on the pericardium is supplied by collateral branches of adjacent vessels. A image in the lung window setting (B) shows about 2.5 cm-sized elongated vascular structure (arrow) in the left upper lobe anterior paramediastinal area. Patch ground-glass-opacities around this are suggestive of aspirated blood.
Since the patient showed persistent hemoptysis, embolization of the supplying vessels was planned. Prior to the embolization of the causative vessels of the hemoptysis, diagnostic aortography, left subclavian arteriography, and left internal mammary arteriography including selective arteriographies of the branches that were directly supplying the vascular mass were performed. During the diagnostic aortography, 2 feeding vessels from left internal mammary artery to vascular mass were identified (Figure 2A), and coil embolization was performed with a 5-Fr catheter and coaxial 2.0-Fr microcatheter (Progreat; Terumo, Tokyo, Japan) (Figure 2B). Microcoils (Tornado embolization microcoil; Cook, Bloomington, IN, USA) were used. From the angiographic findings, multiple systemic arteries supplied the vascular mass lesion and the contrast material drained early to the left pulmonary artery through the fistula in accordance with the CT findings.

Left internal mammary arteriography (A) shows left internal mammary artery to pulmonary artery fistula (arrow) with 2 feeding vessels. After coil embolization of 2 feeders (arrows), the collateral vessels disappeared (B).
During the left subclavian arteriography, the patient underwent a sudden-onset headache due to an unexpected embolic event at the left superior cerebellum through the left vertebral artery. However, after conservative treatment, the headache subsided and patient did not show any neurological deficits or sequelae.
But, patient complained recurrent hemoptysis, so additional diagnostic angiography was performed for searching residual fistulas. In the left subclavian and left internal mammary arteriography, feeding vessels from proximal portion of left subclavian artery were identified (Figure 3A), and residual fistulas from left internal mammary artery were discovered (Figure 3C). And then, coil embolizations to fistulas were performed respectively and the disappearance of them was confirmed (Figure 3B, D).
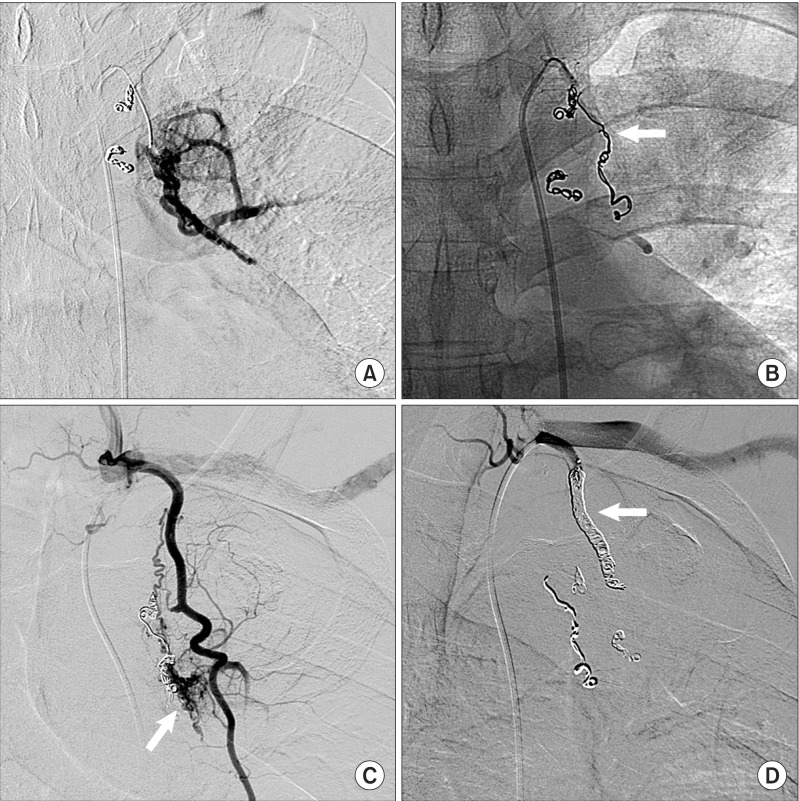
Left subclavian arteriography (A) shows residual systemic artery to pulmonary artery collateral vessels, feed from the proximal portion of left subclavian artery. Coil embolization (arrow) was performed to remove these collateral flows (B). Left internal mammary arteriography (C) shows another collateral vessel from internal mammary artery (arrow). Coil embolization of internal mammary artery (arrow) was performed to prevent hemoptysis (D).
During the diagnostic aortography, left bronchial artery (Figure 4A) and pericardiacophrenic branch of the left inferior phrenic arteries (Figure 4C) were identified as additional supplying branches. For embolization of the left bronchial artery and pericardiacophrenic branch of the left inferior phrenic artery, glue (N-butyl-2-cyanoacrylate) and gelfoam sponge particles were used because these arteries were too small to insert coils (Figure 4B, D).

Abdominal aortography (A) shows connections (arrow) of left pericardiacophrenic branch of inferior phrenic artery with pulmonary artery. After embolization with glue, decreased collateral flow was confirmed (B). Left bronchial arteriography (C) reveals additional collateral vessels from left bronchial artery (lower arrow) to pulmonary artery (upper arrow). After embolization for proximal portion of left bronchial artery (arrow) with gel foam, the collateral vessels are not observed (D).
After the embolization, the patient showed an intermittent old blood clot like sputum due to aspirated blood for several days without any evident hemoptysis. The patient was followed up for 16 months after embolization. During the follow-up period, the patient had no recurrence of hemoptysis.
Discussion
SA-PAF may be classified as either congenital or acquired. However, congenital SA-PAFs in elderly and radiologically evident SA-PAFs without acquired etiology such as infection, trauma, surgery and neoplasm are very rare5. Congenital SA-PAFs may result from functional activation by genetic or external influences of relatively dormant pre-capillary and post-capillary broncho-pulmonary arterial anastomoses found in the normal lung6.
With regard to congenital SA-PAFs, Yu and Chen7 reported an 1.57% incidence of unsuspected systemic-pulmonary collaterals in completely healthy 1-month-old infants with no known predisposing factors. According to Yu and Chen7, the age range was from 1 day to 11 years for 284 cases of congenital SA-PAFs. Our patient had no history of any underlying disease that could be a cause of SA-PAFs. Therefore, the fistulas in this case can be thought of as congenital.
SA-PAFs with more than three feeders are rarely reported. In prior cases, the involved systemic vessels have been various such as the bronchial artery5, internal mammary artery, inferior phrenic artery8, coronary artery9, and so on. In our case, there were multiple systemic arteries supplying the fistula such as the left internal mammary artery, left subclavian artery, pericardiacophrenic branch of left inferior phrenic artery, and left bronchial artery.
The natural course of SA-PAFs is not well known and definite indications for treatment have not been established. Although patients are usually asymptomatic, potential complications maybe possible such as infection (bacterial seeding), hemorrhage (enlargement and rupture), and cardiac complications (pulmonary artery hypertension or congestive heart failure)10. Therefore, several authors have recommended that intervention is necessary for asymptomatic patients because of future risk of endocarditis, angina pectoris, and congestive heart failure11.
Selecting the appropriate therapeutic option is quite controversial. In the past, surgical intervention was the mainstay of treatment since resection of the fistula and the involved lung parenchyma were considered definitive treatment of SA-PAFs. The preferred procedure was lobectomy. Several authors recommended that exploration of the pulmonary artery be carried out for complete closure of the orifice of the fistula to prevent recurrence9,12,13. However, recently, embolization has become the preferred method since major surgery and general anesthesia are unnecessary and the loss of lung parenchyma is minimal. In addition, in the case of SA-PAFs with multiple involved vessels, like in our case, embolization is more useful to control these multiple lesions. Particularly, embolization can be the initial choice for immediate control of hemoptysis.
In our case, during the embolization therapy, there was acute cerebellar infarction; however, no remaining neurologic sequela was observed. Post-embolization neurologic complications have been infrequently reported and usually self-limited14; however, careful observation of neurology should only be necessary.
In conclusion, multiple systemic arteries to pulmonary artery fistulas without any other underlying diseases are very rare, and transcatheter embolization is a reasonable therapeutic option to control bleeding complications.