Mycobacterium intracellulare Pulmonary Disease with Endobronchial Caseation in a Patient Treated with Methotrexate
Article information
Abstract
Methotrexate (MTX) has been established as a standard disease-modifying anti-rheumatic drug. If adequate disease control is achieved for a reasonable period of time, tapering the MTX dosage is recommended because the chronic use of MTX can result in opportunistic infection. We present here a case of a woman with rheumatoid arthritis taking MTX, and the woman developed actively caseating endobronchial Mycobacterium intracellulare disease with pulmonary infiltrations. After discontinuing the MTX, the patient was able to tolerate 18 months of antimycobacterial treatment without flare ups of rheumatoid arthritis, and she completely recovered from nontuberculous mycobacterial respiratory disease.
Introduction
During the past decades, methotrexate (MTX) was regarded as a disease-modifying anti-rheumatic drug due to its effectiveness and safety profile for the treatment of rheumatoid arthritis (RA)1. MTX has been established as the standard disease-modifying anti-rheumatic drug for the treatment of RA2. Infections are common in patients with RA and this may be related either to the underlying RA or to the use of the immunosuppressive drugs, such as MTX2. Nontuberculous mycobacteria (NTM) are ubiquitous bacteria and they have been increasingly detected in both immunocompromized and immunocompetent hosts3. NTM infection may result in an endobronchial infection that is similar to an ordinary tuberculous infection4. Although several cases of the endobronchial lesions caused by NTM infection have been reported5-7, to the best of our knowledge, an actively caseating endobrobchial infection has not been reported in a non-human immunodeficiency virus patient.
We present here an unusual case of actively caseating endobronchial Mycobacterium intracellulare disease in a patient with RA and who developed this infection while receiving MTX.
Case Report
A 58-year old woman was admitted to our hospital with a two month history of a worsening productive cough, fatigue and fever. The patient had a 20 year history of RA involving primarily her hands and wrists, and her disease had become refractory to multiple non-steroidal anti-inflammatory drugs over the past several years. Three year ago, the patient had commenced treatment with MTX at a dose of 12.5 mg per week with concomitant acetaminophen 650 mg twice daily. She had achieved control with these medications. She has never had a similar respiratory problem before. She also complained of mild chest pain, shortness of breath, night sweats and anorexia. The shortness of breath was progressive in nature and it was aggravated by strenuous activities and it subsided when taking a rest.
On physical examination, the patient appeared chronically ill. She was appropriately alert and oriented. She has a body temperature of 37.8℃ and regular heart rhythm with a rate of 80 beats/min. Her blood pressure was 140/80 mm Hg and the respiratory rate was 25 breaths/min. The respiratory system examination was entirely normal except for bibasilar crackles noted on auscultation.
At the time of presentation a complete blood cell count showed a hemoglobin level of 10.9 g/dL and a white blood cell of 6,380 cells/mm3 with 74% neutrophils, and 20% lymphocytes. The other laboratory results were as follows: the erythrocyte sedimentation rate was 28 mm/hr, the C-reactive protein was 6.33 mg/dL, the RA factor was 27.7 IU/mL and the anti nuclear antibody, the anti-neutrophil cytoplasmic antibodies and the anti-ds-DNA were all negative. The anti-human immunodeficiency virus (HIV) antibody was also negative. A chest X-ray revealed multifocal nodular consolidation and ill-defined nodules in both lungs, and this was more prominent in the mid-lung field of the right lung and there was no evidence of mediastinal lymphadenopathy (Figure 1). Bronchoscopy at this time revealed diffuse actively caseating endobronchial lesions in the left main and left upper lobar bronchus (Figure 2). The bronchial biopsy specimen from the left main bronchus showed granulomatous inflammation with caseating necrosis (Figure 2). Ziehl-Neelsen staining of the bronchial washing fluid and expectorated sputum showed acid-fast bacilli (AFB). The polymerase chain reaction test for the specific sequences of M. tuberculosis was negative.

Plain radiography (A) and computed tomography (B) of the chest of a 58-year-old woman who was infected with Mycobacterium intracellulare showed multifocal nodular consolidation and ill-defined nodules in both lungs, and this was all more prominent in the mid-lung field of the right lung, without evidence of mediastinal lymphadenopathy.
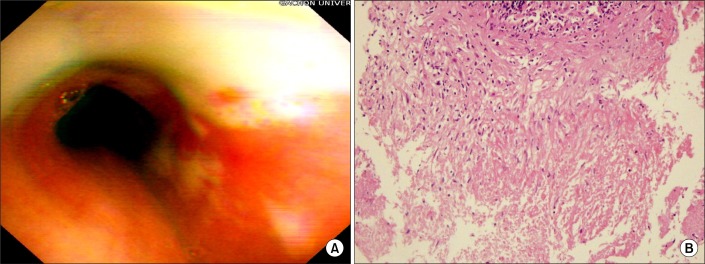
Bronchoscopic photograph of the left main (A) revealed diffuse actively caseating endobronchilal lesions. The bronchial biopsy specimen (B) from the left main bronchi showed granulomatous inflammation with caseating necrosis (H&E stain, ×100).
The MTX was discontinued. Her RA was treated with hydroxychloroquine sulfate 100 mg twice daily, nambumetone 500 mg twice daily and acetaminophen 650 mg twice daily. She was empirically treated with isonizid (300 mg/day), rifampin (600 mg/day), ethambutol (800 mg/day), and pyrazinamide (1,500 mg/day) for tuberculosis pending the AFB culture results. Subsequently, the fever and cough subsided, and the fatigue and shortness of breath regressed. After 6 weeks, the mycobacterium culture of the sputum and bronchial washing fluid yielded M. intracellurare. The isonizid and pyrazinamide were discontinued. Antimycobacterial therapy was continued with rifampicin, ethambutol, and clarithromycin (500 mg twice daily) for 18 months with a good response. Follow up bronchoscopy after 2 months of therapy revealed that the actively caseating endobronchial lesion had completely resolved. At the return visits 3 and 6 months later, the patient was able to do most of her daily activities without respiratory symptoms, and she well tolerated the antimycobacterial medications without any adverse reactions. The chest X-ray checked at the end of the treatment showed normal findings.
At eight months after completing the antimycobacterial medications, the patient was still totally free of respiratory symptoms and the sputum culture for AFB was negative.
Discussion
In this report we have described actively caseating endobronchial NTM disease in a patient with RA and who developed infection while receiving MTX. The patient was found to have actively caseating endobronchial lesions and a pulmonary infiltration due to M. intracellurare. Pulmonary infection caused by M. intracellurare is common in immunocompromised patients such as HIV-infected patients8. Although the specific immunologic dysfunction that predisposes patients to NTM infection is unknown, although defects in interleukin-12 and interferon-γ production are known to increase the risk for disseminated NTM disease in humans9. An exact epidemiologic study on NTM disease has not been reported. However, the recent data has suggested that the prevalence of NTM disease is increasing5. Especially, the popular use of biologic, immune-suppressive therapies for treating patients with RA and other autoimmune inflammatory disease10 might be associated with NTM pulmonary infections4.
Our patient had a clinical picture of fever, dyspnea, cough and night sweats, and her actively caseating endobronchial lesions were different from the obstructive endobronchial lesions of the previously reported cases. The consolidation and nodular shadows were more prominently observed in right-side lung than in left-side lung, but actively caseating lesions were found in left main and left upper lobe bronchus. Endobronchial tuberculosis has been combined in 10% to 20% of patients with active parenchymal disease11. It can be caused by spread of tubercle bacilli within the airway lumen or along peribronchial lymphatic channels from an area of cavitations or tuberculous pneumonia11. Left-side airways are more commonly involved than right-side airways because they are anatomically compressed by the aortic arch, and the left main bronchus is vulnerable to pathologic stricture12. Taken together, these previous reports could explain the reason why the prominent side of pulmonary lesions were different from that of endobronchial lesions in our patient.
Endobronchial lesions caused by NTM infection have already been reported in several studies5-8. The majorities of these patients were HIV-infected and they presented with polypoid masses that caused obstruction of their airways. These lesions responded to antimycobacterial therapy and resection using biopsy forceps6.
In one retrospective study on Pneumocystis jiroveci pneumonia, the number of RA patients with fatal outcome from this infection was higher when they had been treated with MTX as compared to those patients who were not treated with MTX13.
Weekly low doses of oral MTX treatment have become the therapy of choice for patients with RA. There are several reasons for its wide acceptance, including the good disease control, the convenient dosing and the low toxicity compared with other options for antirheumatic therapy1,14. However, despite its wide use along with the increased risk of infection, the previous studies have shown a significant disparity in the prescribing patterns of MTX among physicians1,15. If adequate disease control is achieved for a reasonable period of time, tapering the MTX dosage is recommended because this therapy is not innocuous, and some unwanted drug adverse reactions may arise from the chronic usage of MTX1,14. Because the chronic use of MTX can result in immunologic impairment, doctors who manage patients with this drug should pay attention about the increased risk of opportunistic infection. So, dose reduction or extending the interval for MTX therapy may be a valid therapeutic method after clinical remission, such as in this current patient14. For the current patient, the doses and the interval for MTX had not changed for 3 years. We think that these conditions might have predisposed our patient to NTM infection. Actually, after discontinuing the MTX, the patient was able to tolerate 18 months of antimycobacterial treatment without flare ups of RA, and she completely recovered from the NTM respiratory disease.
In summary, we report here on an unusual case of actively caseating endobronchial M. intracellulare disease with a pulmonary infiltration in a patient with RA, and the patient developed this infection while receiving MTX. According to our findings, NTM infection should be considered in RA patients with chronic usage of the immunosuppressive drugs, such as MTX, especially in proper clinical setting, and prompt diagnosis is important for the effective treatment and prevention of a bronchial stricture.