A Case of Pulmonary Sarcoidosis with Endobronchial Nodular Involvement
Article information
Abstract
Sarcoidosis is a multisystemic disorder of unknown cause that is characterized pathologically by noncaseating granulomas. Diagnosis is based on the exclusion of other infectious, interstitial, and neoplastic diseases and on the typical pathology. Although the lungs and mediastinal lymph nodes are almost involved, endobronchial nodular lesions of sarcoidosis with lung involvements are rare. We report a case of sarcoidosis with lung involvements and endobronchial nodules as confirmed by bronchial biopsy.
Introduction
Sarcoidosis is a cryptogenic general granulomatosis that typically affects adults up to 50 years of age. Sarcoidosis infiltrates all organs, particularly the bilateral hilar lymph nodes, lungs, peripheral lymph nodes, skin, eyes, liver, and spleen1. Boek first used the term 'sarcoid' in 1899 in regard to a condition of multiple tumors and rashes. In Korea, sarcoidosis has occurred in approximately 0.13 out of 100,000 individuals since the first case was reported here in 1984 (The 2nd Nationwide Actual Condition Survey, 2000), but it has recently been on an increasing trend2. The clinical symptoms of sarcoidosis are usually nonspecific and generalized, and include fever, fatigue, anorexia, weight loss, and malaise. The symptoms vary depending upon the organs that are infiltrated. Given that sarcoidosis mostly infiltrates the lungs, respiratory symptoms such as exertional dyspnea, cough, and chest pain are observed in most cases3. In our experience, it is rare that sarcoidosis nodularly infiltrates the bronchus. This is a report of a case of endobronchial nodular sarcoidosis diagnosed by bronchoscopy and a biopsy.
Case Report
A 42-year-old Indian male who was employed as an automotive engineer was referred to our hospital with complaints of a month-long fever and cough. The patient had been diagnosed with pulmonary tuberculosis 5 years previously in India, and he had returned from Thailand a month before visiting our hospital complaining of fever, persistent cough, and general malaise. Before he was referred to our center, he had undergone computed tomography (CT) at another hospital because his symptoms had persisted even after medical treatment, whereon abnormalities had been detected. As noted, the patient had been previously diagnosed with pulmonary tuberculosis by positive mycobacterium tuberculosis culture in April 2003, in India. He completed a 7-month course of anti-tuberculosis medications was completely recovered, and he had no history of diabetes, hypertension, or hepatitis. Blood pressure, pulse, respiratory rate, and temperature were 110/70 mm Hg, 80/min, 20/min, and 36.4℃, respectively. He was alert and oriented to person, place, and time. The cervical lymph nodes and thyroid were not palpable, nor were any noteworthy abnormalities observed on chest, abdominal, or neurologic examinations. On complete blood count hemoglobin level, platelets, and white blood cells count were 14.3 g/dL (rane, 13-18 g/dL), 192,000/mm3 (range, 150,000-450,000/mm3), and 5,880/mm3 (range, 4,000-10,000/mm3) (neutrophils 54.2%, lymphocytes 31.5%, monocytes 9.0%, eosinophils 4.8%). Blood chemistry revealed the following: total protein 7.2 g/dL (range, 6.5-8.3 g/dL), aspartate amino-transferase 23 U/L (range, 5-35 U/L), alanine amino-transferase 17 U/L (range, 5-40 U/L), alkaline phosphatase 201 U/L (range, 66-220 U/L), blood urea nitrogen 11.4 mg/dL (range, 8-20 mg/dL), creatinine 0.97 mg/dL (range, 0.5-1.3 mg/dL), sodium 137 mEq/L (range, 135-150 mEq/L), potassium 4.4 mEq/L (range, 3.3-5.5 mEq/L), calcium 9.24 mg/dL (range, 8.4-10.2 mg/dL), C-reactive protein 5.87 mg/dL (range, 0-5 mg/dL), angiotensin converting enzyme (ACE) 67 IU/L (range, 8-52 IU/L), and urine calcium 4.6 mg/dL. There were no notable abnormalities detected on urinalysis, sputum smear, sputum culture for bacteria and fungus, tubercle bacilli test, or viral and immune serum tests. There was no foreign body in sputum or bronchoalveolar lavage (BAL) fluid.
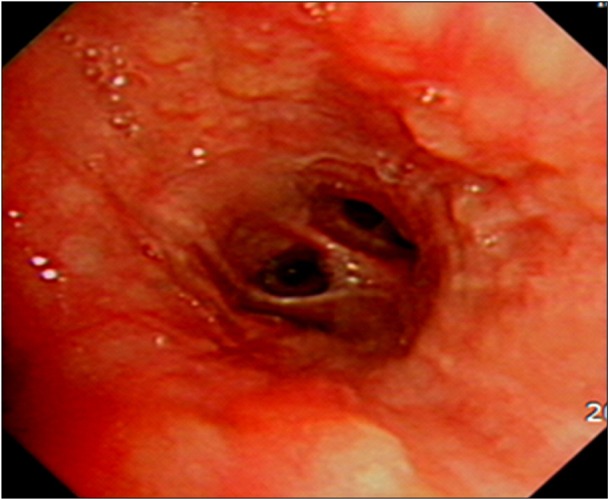
The pulmonary function tests showed a restrictive ventilatory defect with forced vital capacity (FVC) 3.06 L (66% of the predicted value), forced expiratory volume in one second (FEV1) 2.44 L (68% of the predicted value), FEV1/FVC 80%, and DLco 18.6 mL/mm Hg/min (74% of the predicted value). On a plain radiography performed before the patient visited this hospital, nodular lesions were found to be distributed along the bronchi in the right middle and lower lobes (Figure 1), and on the chest CT, thickened lobules and multiple peribronchovascular nodules were observed throughout the entire right lung and in the left lower lobe, in addition to lymphadenopathy around the bronchi and the right pulmonary hilum (Figure 2). The patient's symptoms were relieved to some extent after symptomatic treatment, but they were not eliminated, even during the follow-up. Repeat chest radiography and CT were performed, and the results showed that the known multiple nodules had increased considerably and the hilar lymph nodes had become more enlarged (Figure 3). The patient underwent bronchoscopy, and multiple nodules of different sizes were observed throughout the bronchi (Figure 4). An endobronchial biopsy was performed, whereon multiple non-caseating chronic granulomatous lesions were diagnosed (Figure 5). No acid-fast bacilli and fungus figured out on tissue staining. On BAL, macrophages, lymphocytes, neutrophils, CD4, and CD8 cells were counted as 31%, 68%, 1%, 75.1%, and 16.6%, respectively. Bacteria, fungi, virus, and tubercle bacillus were not detected in the BAL fluid.
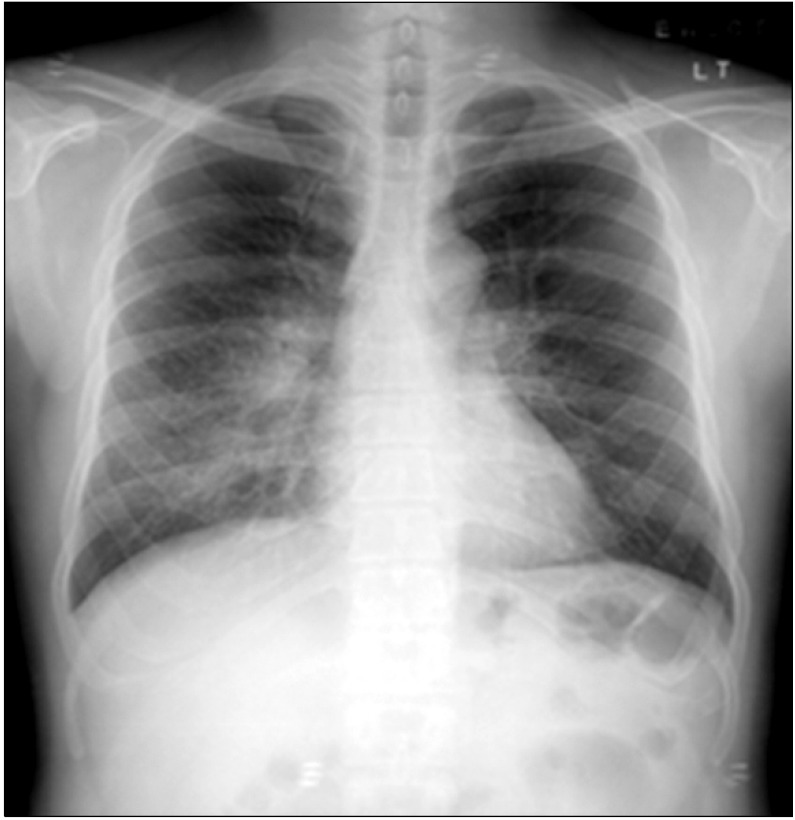
Chest radiograph showing ill-defined nodular peribronchial lesions in both lungs, most prominently in the mid right and lower lung zones.
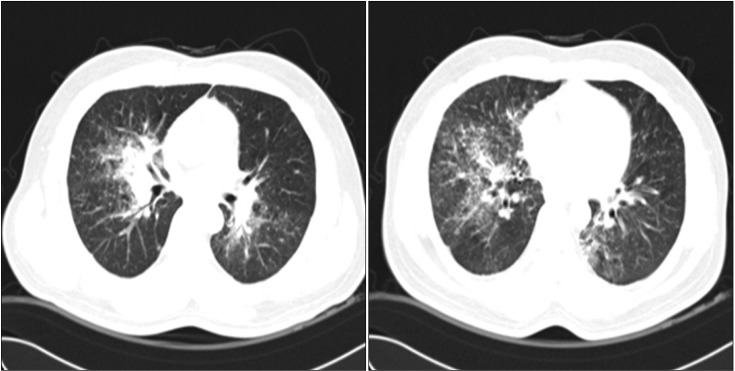
Chest computed tomography showed consolidation along the right upper and intermediate bronchus and small perilymphatic, centrilobular, and bronchovascular nodules with interlobular septal thickening in the upper right, mid, and lower lobes and the lower left lobe.
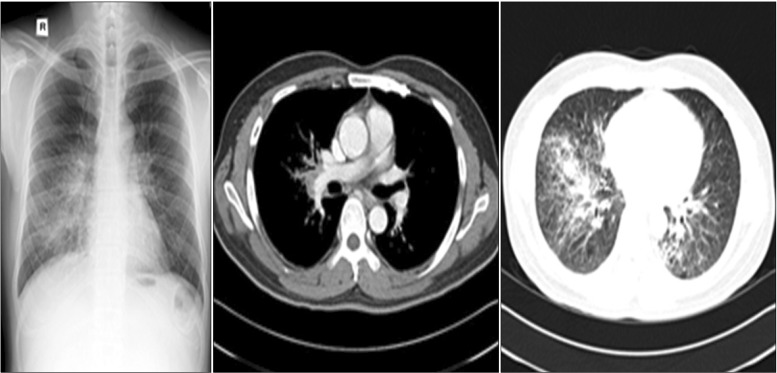
Follow-up chest radiography and computed tomography showed an increased extent of peribronchial infiltration and air space consolidation, interlobular septal thickening, and ill-defined nodules in both lungs, along with increased size of the multiple enlarged hilar and interlobar lymph nodes.
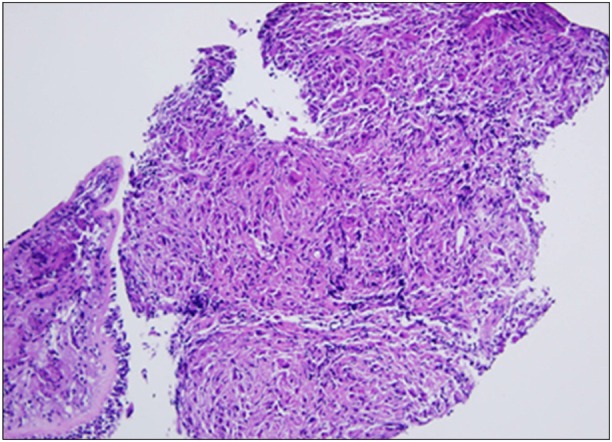
Biopsy of endobronchial nodules showed diffuse granulomatous inflammation with multinucleated giant cells (H&E stain, ×100).
Repeat blood chemistry results included ACE and calcium levels of 94 IU/L and 9.7 mg/dL, respectively. Ophthalmic and dermatologic abnormalities were not detected, and there were no notable abnormalities on echocardiography or gallium-67 scan. Finally, the patient was diagnosed with endobronchial sarcoidosis involving lungs and lymph nodes, and oral prednisolone was prescribed (70 mg; 1 mg/kg/day). The symptoms continued to recede with administration of prednisolone, and the dose was gradually reduced to 5 mg for 7 months. The chest radiography and CT performed at 7 months' follow-up confirmed a remission (Figure 6). Currently, the patient is being monitored with regular follow-up and is not receiving steroids.

Follow-up chest radiography and computed tomography after treatment with oral steroid showed decreased extent of the peribronchial infiltration and air space consolidation in both lungs, along with a significantly decreased extent of the interlobar septal thickening and perilymphatic or ill-defined nodules, and decreased sizes of the multiple enlarged hilar and interlobar lymph nodes.
Discussion
Sarcoidosis is a cryptogenic disease that is characterized by non-caseating chronic granulomas, which infiltrate all organs, including lungs, skin, eyes, bones, nerves, and lymph nodes. The symptoms vary depending upon the organ system affected, but pulmonary infiltration actually comprises more than 90% of cases, and thus, respiratory symptoms such as cough, exertional dyspnea, and chest pain are observed in most cases. Bilateral symmetric hilar or paratracheal lymphadenopathy are cited as salient findings. Approximately 60% of patients will also show an increase in ACE levels, and 10% develop hypercalcemia. Arthritis may occur in 5% to 10% of patients1,4.
It is uncommon that pulmonary sarcoidosis infiltrates the bronchi. When it involves airways, the disease is manifested as mucosal erythema, edema, granularity, and cobblestoning, with plaques, nodules, and bronchial stenosis, airway distortion, traction bronchiectasis, and bronchiolitis. If the disease infiltrates the trachea, main carina, or major bronchi alone, it will occasionally produce significant obstructive symptoms or airway dysfunction.
The diagnosis of sarcoidosis is made based on symptoms, radiographic findings, and tissue biopsy5. It is standard practice to rule out other causes when noncaseating granuloma is found on biopsy. The challenge is that it is difficult to diagnose sarcoidosis in as much as it is similar in clinical, radiological, and histological characteristics to tuberculous and non-tuberculous mycobacterial lung diseases. When it occurs in the bronchus, as in the present case, a biopsy should be performed to distinguish it from malignant diseases such as lung cancer.
Nodules in the upper lobes of the both lungs and bilateral intrathoracic lymphadenopathy are typical radiological findings, but there may be atypical cases, and in such cases, it is difficult to distinguish sarcoidosis from the other similar diseases. In addition, there have been reports of bilateral and multiple peripheral or peribronchovascular non-homogeneous shadows, pulmonary micronodules, or indurated micronodules associated with pulmonary sarcoidosis6,7.
The stages of sarcoidosis are classified by radiological findings. Stage 0 is defined by absence of abnormalities on chest radiography. Stage I includes bilateral hilar lymphadenopathy. In stage II, there is bilateral hilar adenopathy and pulmonary infiltration, and in stage III, the pulmonary infiltration is observed but there is no lymphadenopathy8. Thus, our patient was diagnosed with stage II sarcoidosis, because both adenopathy and pulmonary infiltration were observed.
History and physical exam are crucial to check for sarcoid infiltration of other organs after the sarcoidosis diagnosis. Pulmonary function tests, ophthalmologic exam, gallium scan, and BAL are also necessary, as well as measurements of serum and urine calcium levels and serum ACE9.
Sarcoidosis has a favorable prognosis, and it is reported that 80% to 90% of patients with acute-phase sarcoidosis recover spontaneously10. On the other hand, sarcoidosis will have a poor prognosis when it occurs in patients aged 40 and older, when it lasts for 6 months and longer, when it is not accompanied by erythematous nodules, when lymphadenopathy occurs, when it infiltrates at least 3 organ systems, and when it causes only pulmonary disease11. Explicit uniform treatment guidelines for sarcoidosis have not yet been established. Oral steroids or methotrexate maybe used if sarcoidosis has infiltrated the eyeballs, the nervous system, or the heart at a serious level, or if the patient has severe hypercalcemia, stage II pulmonary disease, or stage II or III symptomatic pulmonary disease, but there are no widely accepted recommendations for dose or duration12.
This patient visited the hospital with a chief complaint of a month-long fever and cough, pulmonary sarcoidosis was suspected on chest radiography, and he was definitively diagnosed with endobronchial nodular sarcoidosis by bronchoscopy with a bronchoscopic biopsy. The patient has achieved complete remission following an extend course (7 months) of oral steroid therapy.
Acknowledgements
This paper was supported by a grant from Wonkwang University in 2011.