 |
 |
| Tuberc Respir Dis > Volume 84(3); 2021 > Article |
|
Abstract
Background
Fractional exhaled nitric oxide (FeNO) is a non-invasive marker for eosinophilic airway inflammation and a good predictor of response to corticosteroids. There is a need for a reliable and accurate measurement method, as FeNO measurements have been widely used in clinical practice. Our study aimed to compare two FeNO analyzers and derive a conversion equation for FeNO measurements in adults.
Methods
We included 99 participants who had chief complaints of chronic cough and difficulty in breathing. The participants underwent concurrent FeNO measurement using NIOX VERO (Circassia AB) and NObreath (Bedfont). We compared the values of the two devices and analyzed their correlation and agreement. We then formulated an equation to convert FeNO values measured by NObreath into those obtained by NIOX VERO.
Results
The mean age of the participants was 51.2┬▒17.1 years, with a female predominance (58.6%). Approximately 60% of the participants had asthma. The FeNO level measured by NIOX VERO (median, 27; interquartile range [IQR], 15-45) was significantly lower than that measured by NObreath (median, 38; IQR, 22-58; p<0.001). There was a strong positive correlation between the two devices (r=0.779, p<0.001). Additionally, Bland-Altman plots and intraclass correlation coefficient demonstrated a good agreement. Using linear regression, we derived the following conversion equation: natural log (Ln) (NObreath)=0.728├ŚLn (NIOX VERO)+1.244.
Exhaled nitric oxide (NO) is an established marker of type 2 airway inflammation in respiratory diseases1,2. Methods for measuring fractional exhaled NO (FeNO) are generally non-invasive, easily accessible, and safe and can be used for diagnosis and monitoring3,4. Various techniques for measuring NO amount have been developed. Nowadays, these devices include chemiluminescence, electrochemical sensors, and laser-based sensors5. Electrochemical analyzers are widely used to measure exhaled NO for clinical studies or clinical practice because they are more user-friendly, cheaper, and portable than chemiluminescence, thereby regarded as the gold standard. Comparison of FeNO levels measured by chemiluminescence and electrochemical analyzers or those equipped with electrochemical devices are already extensively reported6-13. Some studies have also formulated a conversion equation for FeNO values between different devices6-8.
In Korea, several electrochemical analyzers are available, such as NObreath (Bedfont, Kent, UK), NIOX MINO (Circassia AB, Solna, Sweden) and NIOX VERO (Circassia AB) devices. NIOX VERO, which was developed to replace NIOX MINO, has not been fully investigated the relationship with another analyzer called NObreath. In recent studies, FeNO values of NIOX VERO and NObreath were found to be strongly correlated in asthma patients6,12. The median values by NIOX VERO were higher than those of NObreath6,12. For real-world clinical practice, it is important to know whether the devices to measure the level of NO can be comparable with each other. Hence, our study aimed to evaluate the difference and correlation of FeNO levels between NIOX VERO and NObreath. We also sought to derive a conversion equation for FeNO values based on these two devices.
We retrospectively reviewed the medical chart of adult patients aged over 18 years with chief complaints of chronic cough and difficulty in breathing who visited referral clinics. We specifically reviewed patientsŌĆÖ demographics, current respiratory symptoms, smoking history, and lung function test results. The final diagnosis of patients was based on a review of the patientŌĆÖs clinical symptoms, medical history, appropriate diagnostic tests, and/or assessment of the treatment response according to several clinical practice guidelines14-17. We included patients who underwent FeNO measurements both by NIOX VERO (Circassia AB) and NObreath (Bedfont). Finally, 99 patients were enrolled from September 4, 2018 to October 29, 2019. The protocol of this study was reviewed and approved by the institutional review board of our institution (IRB: GFIRB2020-213). The requirement of informed consent was waived because of the retrospective nature of the study.
FeNO levels were measured according to the manufacturerŌĆÖs instructions and the American Thoracic Society/European Respiratory Society (ATS/ERS) guidelines4. Patients underwent two consecutive measurements on each analyzer. We first tested patients with NIOX VERO in the odd number of dates, whereas we first performed FeNO tests with NObreath in the even number of dates. All patients were almost evenly distributed between the first measurement with NIOX VERO (n=47) and NObreath (n=52). Under visible and/or audible feedback, FeNO measurement was performed on each patient. They were instructed to breathe out fully to empty their lungs, close their lips around the mouthpiece on the filter to prevent air leakage, and then inhale deeply to total lung capacity. To maintain a fixed flow rate of 50 mL/sec, they needed to exhale at an exhalation pressure of 10-20 cm H2O consistently. The FeNO levels were repeatedly measured until the two analyzers obtained acceptable values, and all measurements were completed on the same day.
We evaluated patientsŌĆÖ characteristics, spirometric results, and FeNO levels by using chi-square test for categorical variables and ANOVA or Kruskal-Wallis test for continuous variables between different groups. Given that the FeNO levels were nonparametrically distributed, these data were expressed as median with quartiles, and the differences between results by two measures were calculated by Wilcoxon signed-rank test. Other numerical variables were expressed as mean┬▒standard deviation. Using the log-transformed FeNO data, we determined the relationship between devices through PearsonŌĆÖs correlation coefficient (r) and linear regression analysis. Furthermore, we estimated an equation to convert FeNO values measured by NObreath into those obtained by NIOX VERO. The agreement between two different methods of measurement was calculated by plotting the mean intermethod measurement difference, as described by Bland and Altman18. Interdevice agreements were assessed by intra-class correlation coefficient (ICC) and interpreted as follows: ICCŌēź0.75, excellent; 0.60ŌēżICC<0.75, good; 0.40ŌēżICC<0.60, fair; and ICC<0.40, poor correlation19. Statistical data were analyzed using SPSS version 18 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism version 5.0 software (GraphPad Prism Software Inc., San Diego, CA, USA). In addition, p<0.05 was considered to be statistically significant.
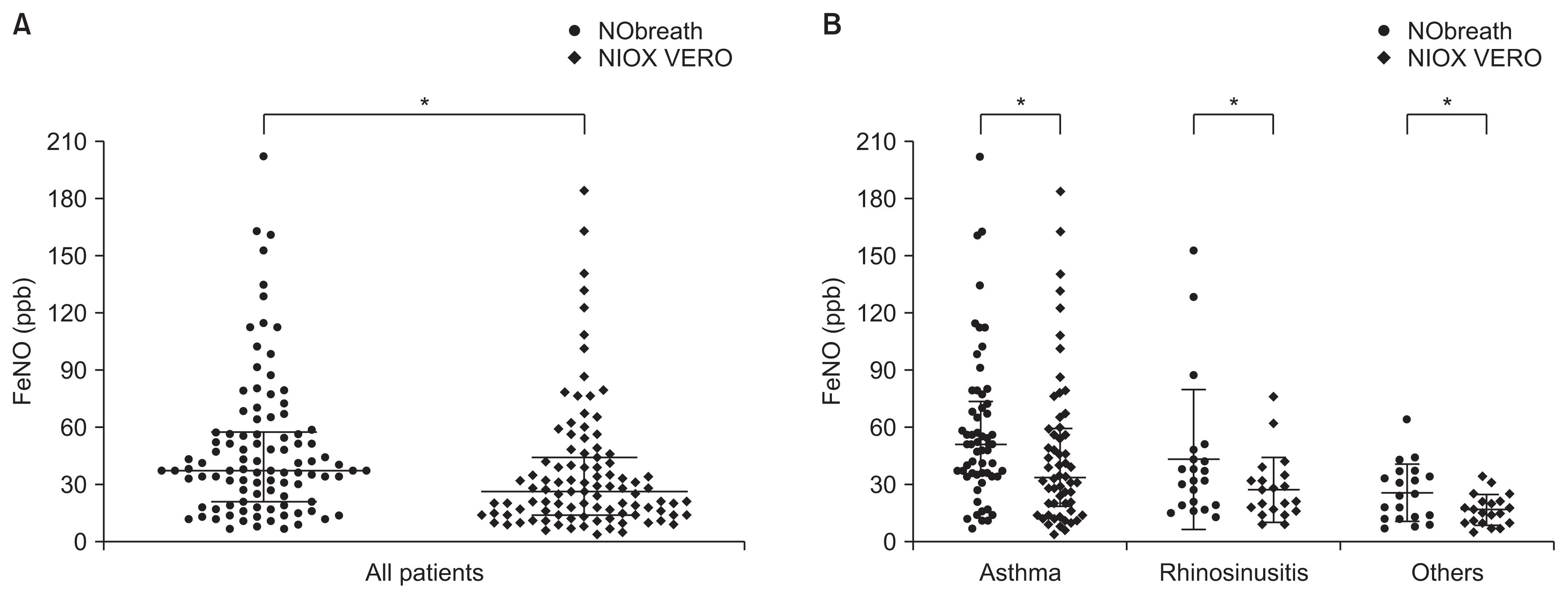
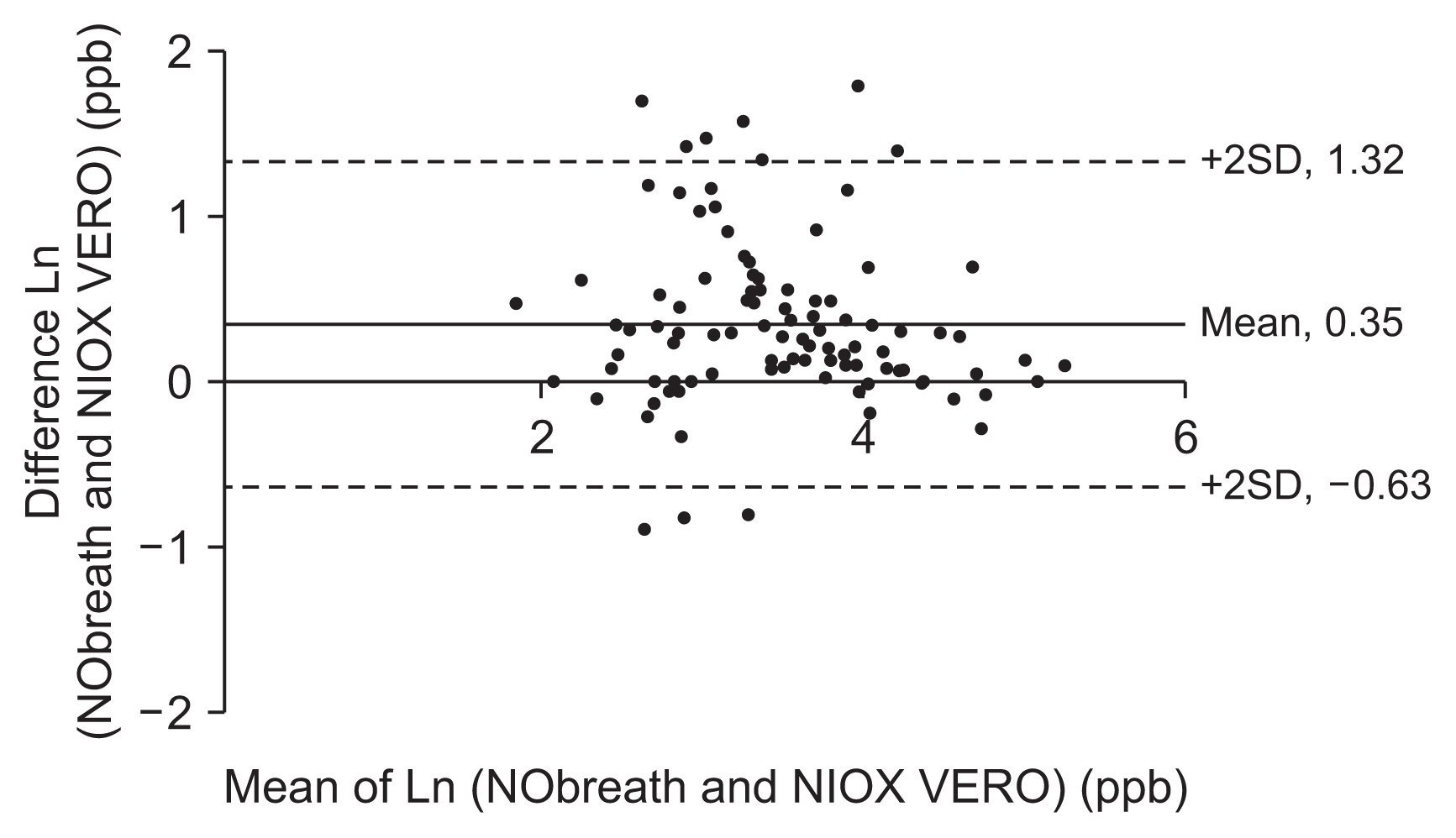
The demographic and clinical characteristics in this study are summarized in Table 1. The mean age was 51 years, with 58 (58.6%) women and 68 (68.7%) nonsmokers. Difficulty in breathing and cough lasting 8 weeks or more were observed in 47 (47.5%) and 52 (52.5%) patients, respectively. Among them, 58 (58.6%) were diagnosed with asthma, 21 (21.2%) had rhinosinusitis, and 20 (20.2%) had other diseases (bronchiectasis [n=1], post-infectious cough [n=1], chronic obstructive pulmonary disease [n=1], unexplained chronic cough [n=2], and unconfirmed diagnosis [n=15]) for chief complaints. The overall mean forced expiratory volume in 1 second (FEV1) value was 84.1%┬▒20.5%, while the FEV1/forced vital capacity (FVC) value was 74.6%┬▒12.9%. Patients with rhinosinusitis and other diseases were mostly female compared with patients with asthma, who had significantly lower percentages of predicted FEV1 values (76.7%┬▒21.9%, p<0.001) and lower FEV1/FVC values (70.7%┬▒14.8%, p=0.001). A comparison of values obtained using the two analyzers is shown in Figure 1. Overall, the median (interquartile range [IQR]) values using NIOX VERO (median, 27.0; IQR, 15.0-45.0) were significantly lower than those obtained by NObreath (median [IQR], 38.0 [22.0-58.0]; p<0.001). The same results were observed among patients with asthma (median [IQR], 52.0 [35.8-74.3] vs. 34.5 [19.3-60.3]; p<0.001), those with rhinosinusitis (median [IQR], 33.0 [20.0-46.5] vs. 22.0 [16.0-34.5]; p=0.005), and those with other diagnoses (median [IQR], 25.5 [13.3-37.3] vs. 17.0 [11.0-22.0]; p=0.004). NObreath levels strongly positively correlated with NIOX VERO levels (r=0.779, p<0.001) (Figure 2). Using these results, we determined regression equations and calculated the estimated FeNO level as follows: natural log (Ln) (NObreath)=0.728├ŚLn (NIOX VERO)+1.244. The estimated values calculated by this equation are displayed in Table 2. The Bland-Altman plot revealed a good agreement between the devices, with nonproportional bias. The mean interdevice difference in Ln (FeNO) was 0.35 ppb, and the 95% limits of agreement were ŌłÆ0.63 and 1.23 ppb (Figure 3). Moreover, the ICC between FeNO measurements during this study was excellent (ICC, 0.88; 95% limit of agreement, 0.81-0.92).
This study compared the FeNO values obtained by two different analyzers in an adult population with respiratory symptoms. The FeNO levels measured by NObreath were approximately 40% higher than those measured by NIOX VERO. A similar pattern was also seen in not only patients diagnosed with asthma, rhinosinusitis, and other diagnoses but also smoking status and sex (data not shown). Two previous studies In Japan directly compared NIOX VERO and NObreath among patients with asthma6,12. In contrast to our findings, NIOX VERO yielded higher values than NObreath in both children (n=88) and adult participants (n=44)6,12. In other previous studies, NObreath in healthy adults or in adult patients with asthma revealed higher mean values than NIOX MINO, while an opposite trend was observed among children with asthma20,21. FeNO levels by NIOX MINO were slightly higher than those of NIOX VERO in an asthma population22. Therefore, the difference in FeNO values requires further investigation with a larger cohort of the target population. The correlation between measurements across all devices has been reported variably7,8,23. FeNO measurements of different devices in adults and unspecified age groups displayed a close correlation, ranging from 0.68 (n=18, p<0.001) in healthy adults to 0.94 (n=1,369, p<0.001) in adults with asthma, and 0.95 (n=154, p<0.001) in subjects with asthma aged 14-83 years7,8,23. These analyzer differences can be attributed to the variations in calibration gases or measurement procedures, although both devices were calibrated according to the manufacturerŌĆÖs instructions and the ATS/ERS recommendations4.
In this study, all measurements revealed a strong correlation between the two analyzers, and the correlation obtained in patients with asthma was high, reaching 0.83 (n=58, p<0.001), consistent with the results in previous studies7,8,23. Given the positive correlation, a linear equation can be derived to estimate the values of NObreath according to the FeNO levels measured by NIOX VERO. Compared with the FeNO levels of above 50 ppb measured by NIOX VERO, those of NObreath derived from the conversion equation were above 60 ppb.
Conversely, when the NObreath levels from the conversion equation were set at 25 ppb, the measured NIOX VERO levels were below 25 ppb. These findings would lead to the overestimation or underestimation of airway inflammation, depending on the devices employed. Hence, in clinical practice, the FeNO level results obtained by various analyzers require careful consideration when interpreting the findings. Nonetheless, this conversion equation can be beneficial for interpreting the FeNO levels measured by NIOX VERO and NObreath. We suggest conducting a comprehensive assessment considering the clinical features and treatment effects. Relative reliability and absolute reliability were estimated using the Bland-Altman plot and the ICC, which are statistical methods essential for analyzing agreement between different variables in the same group18,19. The levels of agreement are varied between studies and between comparator devices. Previous studies involving an adult population observed a range of 95% limits of agreement of approximately 10 ppb9,13. Our study revealed that NIOX VERO and NObreath had a good interdevice agreement without proportional error, and the reference limits were equal to 2 ppb in the arithmetic scale. Further research is warranted to determine reliability between these devices by conducting appropriate sample size calculations, prespecifying clinically important limits of agreements, and thoroughly examining both measurements. This study has certain limitations that must be addressed. First, this study is a single-center study describing adult patients. Results can be affected by selection bias and may not be applicable to children with asthma and other diseases. Second, our study did not conduct detailed analyses of factors that had introduced measurement differences, and healthy subjects were not included in our study. Lastly, serial breathing maneuvers and the method of exhaled NO may have a profound effect of exhaled NO levels 10. In NIOX VERO, patients should exhale through the filter while keeping the cloud within the limits shown by the white lines on the screen. When patients did not perform the steps correctly, NIOX VERO devices displayed an error message and patients need to start all over from the beginning. In NObreath, patients should exhale through the filter while keeping a ball in the middle of the white band in the flow indicator. The measurement with NObreath was performed up to 3 times to get the correct values. In this study, we performed more tests with NIOX VERO (three or more attempts) than NObreath (within two attempts) to find the appropriate levels. Additional studies are required to overcome these limitations and better elucidate whether the equality of diagnostic accuracy would be achievable with all devices.
In conclusion, the FeNO levels measured by NIOX VERO and NObreath were significantly different but strongly correlated and in good agreement. These findings and their associated conversion equations may provide useful information to guide clinicians in interpreting the FeNO levels with different devices.
Notes
AuthorsŌĆÖ Contributions
Conceptualization: Kang SY, Lee SM, Lee SP. Methodology: Kang SY, Lee SM. Formal analysis: Kang SY. Data curation: Kang SY. Investigation: Kang SY, Lee SM, Lee SP. Writing - original draft preparation: Kang SY. Writing - review and editing: Kang SY, Lee SM, Lee SP. Approval of final manuscript: all authors.
Acknowledgments
We would like to thank Mil Hwang, RN and Miji Lee, RN for their help and support in conducting study.
Figure┬Ā1
Comparison of FeNO levels between the two analyzers: all patients (A) and patients diagnosed with asthma, rhinosinusitis, and other diagnoses (B). *Significant differences between two analyzers. FeNO: fractional exhaled nitric oxide; ppb: parts per billion.
Figure┬Ā2
Correlation between the FeNO values obtained with the two analyzers. Ln: natural logarithm; ppb: parts per billion.

Figure┬Ā3
A Bland-Altman plot for evaluating the agreement between the two analyzers. Ln: natural logarithm; ppb: parts per billion; SD: standard deviation.
Table┬Ā1
Demographic and clinical characteristics of the study subjects
| Characteristic | All (n=99) | Asthma (n=58)* | Rhinosinusitis (n=21)* | Others (n=20) | p-value |
|---|---|---|---|---|---|
| Age | 51.19┬▒17.10 | 50.72┬▒17.30 | 51.95┬▒18.42 | 51.75┬▒15.84 | 0.949 |
| Male/female | 41 (41.4)/58 (58.6) | 30 (51.7)/28 (48.3) | 5 (23.8)/16 (76.2) | 6 (30.0)/14 (70.0) | 0.036 |
| Smoking history | 0.139 | ||||
| ŌĆāNon-smoker | 68 (68.7) | 34 (58.6) | 19 (90.5) | 15 (75.0) | |
| ŌĆāEx-smoker | 15 (15.1) | 13 (22.4) | 0 (0) | 2 (10.0) | |
| ŌĆāCurrent smoker | 16 (16.2) | 11 (19.0) | 2 (9.5) | 3 (15.0) | |
| BMI, kg/m2 | 24.30┬▒4.39 | 24.67┬▒4.76 | 24.11┬▒3.51 | 23.41┬▒4.13 | 0.533 |
| Chief complaints | 0.332 | ||||
| ŌĆāDifficulty breathing | 47 (47.5) | 33 (56.9) | 3 (14.3) | 11 (45.0) | |
| ŌĆāChronic cough | 52 (52.5) | 25 (43.1) | 18 (85.7) | 9 (55.0) | |
| FEV1, % predicted | 84.10┬▒20.49 | 76.71┬▒21.92ŌĆĀ,ŌĆĪ | 95.48┬▒9.75 | 93.60┬▒14.81 | <0.001 |
| FEV1/FVC, % | 74.58┬▒12.89 | 70.67┬▒14.77ŌĆĀ,ŌĆĪ | 79.43┬▒5.11 | 81.16┬▒7.23 | 0.001 |
| FeNO NObreath, median ppb (IQR) | 38.00 (22.00-58.00) | 52.00 (35.75-74.25)ŌĆĀ,ŌĆĪ | 33.00 (20.00-46.50) | 25.50 (13.25-37.25) | <0.001 |
| FeNO NIOX VERO, median ppb (IQR) | 27.00 (15.00-45.00) | 34.50 (19.25-60.25)ŌĆĪ | 22.00 (16.00-34.50)┬¦ | 17.00 (11.00-22.00) | <0.001 |
Table┬Ā2
Estimated values calculated with the derived conversion equation*
| NIOX VERO (ppb) | NObreath (ppb) |
|---|---|
| 5 | 11 |
| 10 | 19 |
| 15 | 25 |
| 20 | 31 |
| 25 | 36 |
| 30 | 41 |
| 35 | 46 |
| 40 | 51 |
| 45 | 55 |
| 50 | 60 |
| 55 | 64 |
| 60 | 68 |
References
1. Alving K, Weitzberg E, Lundberg JM. Increased amount of nitric oxide in exhaled air of asthmatics. Eur Respir J 1993;6:1368-70.


2. Gustafsson LE, Leone AM, Persson MG, Wiklund NP, Moncada S. Endogenous nitric oxide is present in the exhaled air of rabbits, guinea pigs and humans. Biochem Biophys Res Commun 1991;181:852-7.


3. Song WJ, Kwon JW, Kim EJ, Lee SM, Kim SH, Lee SY, et al. Clinical application of exhaled nitric oxide measurements in a Korean population. Allergy Asthma Immunol Res 2015;7:3-13.


4. American Thoracic Society; European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med 2005;171:912-30.


5. Maniscalco M, Vitale C, Vatrella A, Molino A, Bianco A, Mazzarella G. Fractional exhaled nitric oxide-measuring devices: technology update. Med Devices (Auckl) 2016;9:151-60.



6. Inoue Y, Sato S, Manabe T, Makita E, Chiyotanda M, Takahashi K, et al. Measurement of exhaled nitric oxide in children: a comparison between NObreath(R) and NIOX VERO(R) analyzers. Allergy Asthma Immunol Res 2018;10:478-89.



7. Tanabe Y, Harada N, Ito J, Matsuno K, Takeshige T, Harada S, et al. Difference between two exhaled nitric oxide analyzers, NIOX VERO((R)) electrochemical hand-held analyzer and NOA280i((R)) chemiluminescence stationary analyzer. J Asthma 2019;56:167-72.

8. Pisi R, Aiello M, Tzani P, Marangio E, Olivieri D, Chetta A. Measurement of fractional exhaled nitric oxide by a new portable device: comparison with the standard technique. J Asthma 2010;47:805-9.


9. Khalili B, Boggs PB, Bahna SL. Reliability of a new hand-held device for the measurement of exhaled nitric oxide. Allergy 2007;62:1171-4.


10. Korn S, Wilk M, Voigt S, Weber S, Keller T, Buhl R. Measurement of fractional exhaled nitric oxide: comparison of three different analysers. Respiration 2020;99:1-8.


11. Menzies D, Nair A, Lipworth BJ. Portable exhaled nitric oxide measurement: comparison with the ŌĆ£gold standardŌĆØ technique. Chest 2007;131:410-4.


12. Tsuburai T, Kamide Y, Nakamura Y, Tomita Y, Hamada Y, Watai K, et al. Differences in fraction of exhaled nitric oxide values measured by two hand-helded analyzers (NObreath and NIOX Vero). Arerugi 2017;66:204-8.

13. Alving K, Janson C, Nordvall L. Performance of a new handheld device for exhaled nitric oxide measurement in adults and children. Respir Res 2006;7:67.



14. Brozek JL, Bousquet J, Agache I, Agarwal A, Bachert C, Bosnic-Anticevich S, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines-2016 revision. J Allergy Clin Immunol 2017;140:950-8.

15. Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012: a summary for otorhinolaryngologists. Rhinology 2012;50:1-12.


16. Morice AH, Millqvist E, Bieksiene K, Birring SS, Dicpinigaitis P, Domingo Ribas C, et al. ERS guidelines on the diagnosis and treatment of chronic cough in adults and children. Eur Respir J 2020;55:1901136.


17. Global strategy for asthma management and prevention [Internet] Fontana, WI: Global Initiative for Asthma; 2020 [cited 2020 Oct 15]. Available from: http://www.ginasthma.org
.
18. Bland JM, Altman DG. Comparing methods of measurement: why plotting difference against standard method is misleading. Lancet 1995;346:1085-7.


19. Cicchetti DV. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol Assess 1994;6:284-90.

20. Fukuhara A, Saito J, Sato S, Sato Y, Nikaido T, Saito K, et al. Validation study of asthma screening criteria based on subjective symptoms and fractional exhaled nitric oxide. Ann Allergy Asthma Immunol 2011;107:480-6.


21. Kapande KM, McConaghy LA, Douglas I, McKenna S, Hughes JL, McCance DR, et al. Comparative repeatability of two handheld fractional exhaled nitric oxide monitors. Pediatr Pulmonol 2012;47:546-50.


-
METRICS

- Related articles
-
Measurement of Fractional Exhaled Nitric Oxide in Stable Bronchiectasis2013 January;74(1)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Print
Print Download Citation
Download Citation



