Introduction
Breast cancer is one of the most prevalent cancer in women and can result in various thoracic manifestations from either treatment, its complications or tumor recurrence and metastasis1. Thus, the accurate diagnosis of various thoracic manifestations in patients with breast cancer is critical to guide further treatment. The lung is a common site of metastasis and it may present with solitary or multiple nodules, lymphangitic metastasis, or airspace consolidation2. However, ground glass opacity (GGO) is a rare form of metastatic lung involvement and can be misdiagnosed as drug-induced pneumonitis or viral infection. Here, along with a literature review, we report the case of a 43-year-old woman with breast cancer that metastasized to the lung and presented as bilateral GGO on chest computed tomography (CT).
Case Report
A 43-year-old woman was referred to our clinic with fever and persistent cough and sputum for two weeks. She had been diagnosed with breast cancer (invasive ductal carcinoma, estrogen receptor negative, progesterone receptor negative, and human epidermal growth factor receptor 2 negative, cT2N2M0, stage IIB) with axillary lymph node metastasis three months prior to presentation and was on neoadjuvant chemotherapy. After completing four cycles of AC chemotherapy (doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2 on day 1 every three weeks), she was currently on the second cycle of docetaxel/S-1 chemotherapy (docetaxel 75 mg/m2 and S-1 30 mg/m2 twice daily for 14 days every three weeks) as part of a clinical trial at our institution. It was her ninth day of chemotherapy when she visited the clinic. The patient, a non-smoker with no known allergies, had no other past medical history, and denied hazardous substance exposure or recent travel.
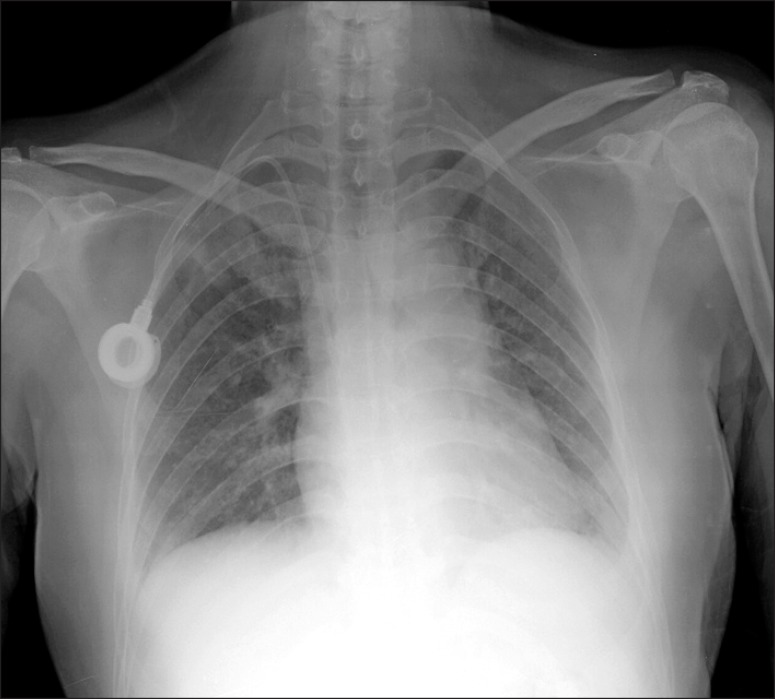
She complained of cough with sputum production and mild dyspnea. She had a low-grade fever of 37.7Ōäā and breath sounds were almost silent on auscultation. The chest radiograph showed a suspicious consolidation in the right upper lung field and diffuse bilateral haziness (Figure 1). Laboratory data revealed no leukocytosis (white blood cell [WBC] count, 5,590/┬ĄL), serum chemistry values were within normal limits, and C-reactive protein was elevated (221.18 mg/L). High-resolution chest CT showed patchy consolidations and GGO in the subpleural area of both upper lobes and diffuse GGO bilaterally in the lower lobes (Figure 2). The primary tumor in the left breast remained stable and the metastatic lymph node in the left axilla seemed to be decreased in size after the aforementioned six cycles of chemotherapy.
Initially, she was thought to have bacterial or viral pneumonia, given immunosuppression from chemotherapy, or drug-related interstitial pneumonitis, and was treated with antibiotics for three weeks. However, the bilateral lung GGO showed no change on serial follow-up CT scans. Fiberoptic bronchoscopy was done and bronchoalveolar lavage fluid from the right upper lobe was sent for analysis. It contained 1,000 WBCs/mL with 98% macrophages and 2% lymphocytes. Cultures revealed no specific pathogen, and cytology showed no evidence of malignant cells. After empiric treatment with steroids (35 mg of prednisolone) for seven days, her symptoms showed mild improvement, but the diffuse infiltrative lung lesions remained unchanged.
While she was being treated for suspected interstitial pneumonia and with the chemotherapy delayed, changes in the skin color of the breast were observed. Considered to be a clinical sign of disease progression, neoadjuvant chemotherapy was stopped after six cycles out of the initially-planned eight and she dropped out of the clinical trial. Magnetic resonance imaging of the breast showed no change in the maximum extent of the primary tumor in the left breast and the metastatic lymph node in the left axilla, but showed an increase in size of the subareolar mass with breast contraction, suggesting disease progression. Thus, she underwent modified radical mastectomy and axillary lymph node dissection for the underlying breast cancer, and thoracoscopic lung biopsy of the right upper lobe and right middle lobe was performed concurrently for the pathologic diagnosis of lung involvement. Postoperative pathology of the lung revealed metastatic carcinoma from the breast, predominantly in the lymphovascular spaces (Figure 3). Diagnosed with breast cancer with lung metastasis, she is currently undergoing palliative chemotherapy.
Discussion
Lung metastasis exhibiting diffuse GGO is a very rare pattern and can mimic interstitial lung disease (ILD). Especially in this patient, considering the history of recent chemotherapy with agents with reported pulmonary toxicity3-5, diffuse lung infiltrates and GGO might lead to a diagnosis of drug-induced pneumonitis or viral infection. Thoracoscopic lung biopsy revealed metastatic carcinoma in the lymphovascular space in this case.
Breast cancer is the second most common cancer in women in Korea, and the incidence and the mortality of breast cancer have continuously increased over the last ten years6. Changes in thoracic imaging in patients with breast cancer can result from treatment, treatment-related complications, or metastases. Some pulmonary conditions may need specific treatment such as antibiotics or steroids. Also, the presence of lung metastasis is a critical factor in deciding treatment options for breast cancer. Thus, the accurate diagnosis of thoracic manifestations in patients with breast cancer is of great importance.
Chemotherapy agents can cause lung injury either by direct cytotoxicity or immune-mediated reactions7. Clinical manifestations of chemotherapy-related lung injury are very nonspecific and many times it is difficult to differentiate them from other pulmonary conditions such as pulmonary edema, interstitial pneumonia, or pulmonary hemorrhage. Common radiologic features of drug-induced lung diseases include GGO, consolidation, interlobular septal thickening, and centrilobular nodules8. Cyclophosphamide and docetaxel are known to have pulmonary toxicity and to cause hypersensitivity pneumonitis3,4, and doxorubicin-related lung injury has been reported previously5. However, the diagnosis is usually one of exclusion, and is based on the temporal relationship with chemotherapy.
Lymphangitic metastasis is the most frequently observed pattern of pulmonary metastasis from breast cancer9. Lymphangitic lung metastasis of breast cancer usually appears as reticular or reticulonodular interstitial markings, or interlobar septal thickening (Kerley B lines) on imaging1. Other common patterns of pulmonary metastases include multiple pulmonary nodules or a solitary pulmonary nodule, which results from hematogenous tumor spread. The tumor can spread along the alveolar walls in a pattern like that of bronchioloalveolar carcinoma (BAC) and form airspace infiltrations that can mimic pneumonia or pneumonitis1. In addition, in malignancies other than breast cancer, various atypical patterns of radiographic findings have been reported, including air-space consolidation2, bilateral ground glass opacities10, and diffuse interstitial infiltrates11.
Ground glass opacities of the lung can represent various entities such as pneumonia, pneumonitis, lymphoproliferative diseases, or fibrosis. Persistent nodular GGOs are known to be related to premalignant or malignant conditions including atypical adenomatous hyperplasia, BAC, and invasive adenocarcinoma12. In the case of diffuse ground glass opacities, it can be associated with various inflammatory and infectious conditions, including ILDs. One study investigated the diagnostic accuracy of high-resolution CT in diffuse lung diseases, and GGO as a predominant pattern was the most unreliable (44.2% to 75.5%) when compared with honeycombing, bronchovascular thickening (more than 90%), and cysts (80% to 89%) as a predominant pattern13. All of the clinical information, laboratory results, and radiographic studies has to be considered, but in the absence of a confident clinical diagnosis and with the possibility that definitive diagnosis by lung biopsy might change treatment plans, lung biopsy is recommended for the diagnosis of ILD14.
Lung biopsy is considered when there are unexplained signs and symptoms with atypical radiographic features that are usually progressive or rapidly deteriorating. Indications of lung biopsy in evaluating patients with suspected ILD include the followings: to prove a specific diagnosis, to exclude malignancy, or to identify certain disease entities that need specific treatments such as antibiotics or steroids. Since lung biopsy, either transbronchial or surgical, is associated with morbidity and mortality even in small numbers, the risks and benefits of the procedure should be carefully weighed before pursuing it. Transbronchial lung biopsy (TBLB) is associated with 1-4% of reported complications, of which pneumothorax and hemorrhage are most common, and approximately 0.1% of mortality with hemorrhage the main cause. In case of surgical lung biopsy, the complication rate is up to 7% with surgery related mortality rate of less than 1%14. With video-assisted thoracoscopic surgery increasingly used, surgical lung biopsy has become less invasive in recent years. Diagnostic yield of TBLB and surgical lung biopsy is reported to be 29-79% and 37-100%, respectively14. Lung biopsy should be performed when the possible benefits of definitive histopathologic diagnosis and treatment are expected to overweigh the potential risks associated with the procedure. The decision has to be made on a case by case basis.
The diagnosis of suspected ILD has always been difficult but with comprehensive clinical information, laboratory results, and the aid of high-resolution CT, a presumptive diagnosis can be made without lung biopsy in many cases15. However, as in this case, metastatic lung cancer can also appear as ground glass opacities, mimicking interstitial lung disease. Lung metastasis should always be considered in interstitial lung diseases, especially when the patient has a prior history of malignancy, and lung biopsy should be performed in the absence of a confident clinical diagnosis.
We report a case of metastatic lung cancer presenting as diffuse ground glass opacities, mimicking interstitial lung disease. Malignancy should always be considered in the differential diagnosis of interstitial lung disease, and lung biopsy is required to exclude it.




 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Print
Print Download Citation
Download Citation






