A Critical Systematic Review for Inhaled Corticosteroids on Lung Cancer Incidence: Not Yet Concluded Story
Article information
Abstract
Background
To systematically review studies on inhaled corticosteroids (ICS) and lung cancer incidence in chronic airway disease patients.
Methods
We conducted electronic bibliographic searches on OVID-MEDLINE, EM-BASE, and the Cochrane Database before May 2020 to identify relevant studies. Detailed data on the study population, exposure, and outcome domains were reviewed.
Results
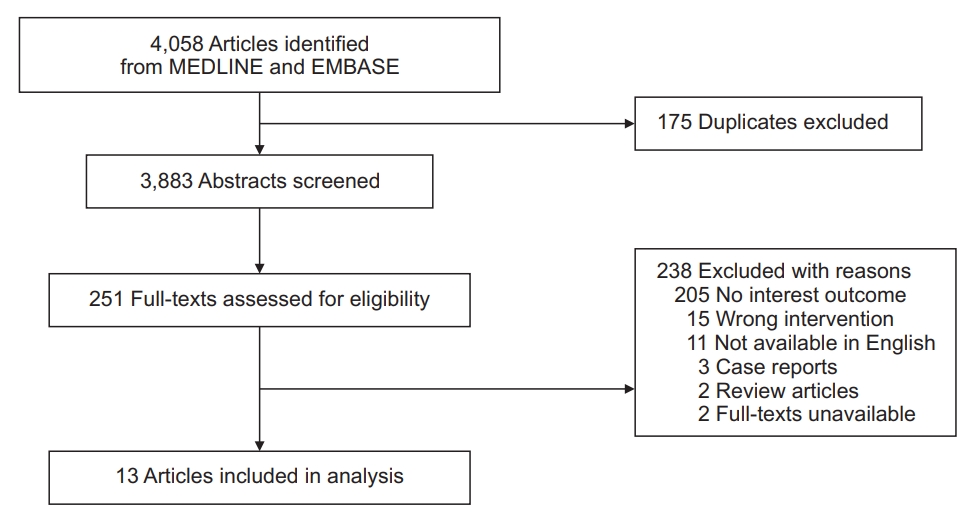
Of 4,058 screened publications, 13 eligible studies in adults with chronic obstructive pulmonary disease (COPD) or asthma evaluated lung cancer incidence after ICS exposure. Pooled hazard ratio and odds ratio for developing lung cancer in ICS exposure were 0.81 (95% confidence interval, 0.64 to 1.02; I2=95.7%) from 10 studies and 1.02 (95% confidence interval 0.50 to 2.07; I2=94.7%) from three studies. Meta-regression failed to explain the substantial heterogeneity of pooled estimates. COPD and asthma were variously defined without spirometry in 11 studies. Regarding exposure assessment, three and 10 studies regarded ICS exposure as a time-dependent and fixed variable, respectively. Some studies assessed ICS use for the entire study period, whereas others assessed ICS use for 6 months to 2 years within or before study entry. Smoking was adjusted in four studies, and only four studies introduced 1 to 2 latency years in their main or subgroup analysis.
Conclusion
Studies published to date on ICS and lung cancer incidence had heterogeneous study populations, exposures, and outcome assessments, limiting the generation of a pooled conclusion. The beneficial effect of ICS on lung cancer incidence has not yet been established, and understanding the heterogeneities will help future researchers to establish robust evidence on ICS and lung cancer incidence.
Introduction
Inhaled corticosteroids (ICSs) are milestones in the pharmaceutical treatment of chronic airway diseases. According to asthma treatment guidelines, ICSs are recommended as essential agents for asthma control [1]. The use of ICSs in patients with chronic obstructive pulmonary disease (COPD) has also been evaluated over the past decade [2], and they reduced acute exacerbation and improved lung function and quality of life when combined with inhaled bronchodilators in patients with severe COPD [3]. ICSs reduce airway inflammation, especially eosinophilic inflammation [4].
Studies have reported that the use of ICS may reduce the risk of lung cancer in airway diseases [5]. These findings are promising; however, further considerations are needed before accepting the results from the following points of view. Most studies were not randomized controlled trials, and the criteria for the study population, exposure, and outcome measures varied. Recently, two similar studies meta-analyzed the outcomes of relevant publications and concluded that ICS reduced the occurrence of lung cancer [6,7]. However, a meta-analysis can only be applied when relevant publications are sufficiently homogeneous in the patients, intervention, comparators, and outcomes (PICO) domain of relevant studies. When candidate publications for pooling are substantially heterogeneous, a mathematical pooling of outcomes can misguide conclusions. Thus, this study aimed to systematically review original studies on ICS and lung cancer incidence in patients with chronic airway diseases, along with provisional pooling, and propose potential standards to be applied to future relevant research.
Materials and Methods
This systematic review was performed in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline [8]. The study protocol was registered in the International Prospective Register of Systematic Reviews (ID: CRD42019142541). This meta-analysis handled data extracted from relevant published studies and did not require Institutional Review Board approval.
1. Search strategy
We searched the OVID-MEDLINE, EMBASE databases, and Cochrane Database of Systematic Reviews to identify relevant original publications dealing with the protective effect of inhaled (keywords: aerosol, nebulizer, inhalation, or with spacer) corticosteroids (keywords: corticosteroid or glucocorticoid or beclomethasone or betamethasone or budesonide or clobetasol or dexamethasone or fluprednisolone or methylprednisolone or triamcinolone) on lung cancer incidence (keywords: lung cancer) in patients with obstructive airway diseases (keywords: COPD or emphysema or asthma). The initial search was conducted on June 18, 2019, limited to English publications, and was updated on May 26, 2020. The authors reviewed the literature to supplement the search strategy of this study.
2. Study selection
Two authors independently screened the search results by title and abstract and subsequently reviewed the full-text articles using the following eligibility criteria: (1) adult study population with COPD or asthma; (2) studies or subsets evaluating lung cancer incidence after ICS exposure compared to those not exposed to ICS; and (3) data described in sufficient detail to extract outcomes as the odds ratio (OR) or hazard ratio (HR). We included either prospective or retrospective randomized controlled trials, observational cohort studies, or case-control studies. Case reports, review articles, guidelines, phantom studies, animal studies, letters, editorials, and abstracts were excluded. Any discrepancies between the authors were resolved by consensus.
3. Data extraction and quality assessment
Two authors independently extracted data from the included studies using a standardized Excel form. The following data were extracted: (1) study characteristics; (2) demographic characteristics of the study population; (3) information about how to define ICS users and steroid doses; and (4) outcome information, including the mean or median follow-up period of observation and lung cancer incidence in patients depending on ICS exposure.
The Newcastle-Ottawa Quality Scale for cohort studies was used to assess the quality of the included studies [9]. The scale comprises a maximum of 4 points for the selection domain, 2 points for the comparability domain, and 3 points for the exposure or outcome domain. Scores of 7 or higher and 5–6 indicated high-quality and moderate-quality studies, respectively [10]. Two reviewers independently reviewed the studies, and any discrepancies were resolved through discussion.
4. Meta-analysis
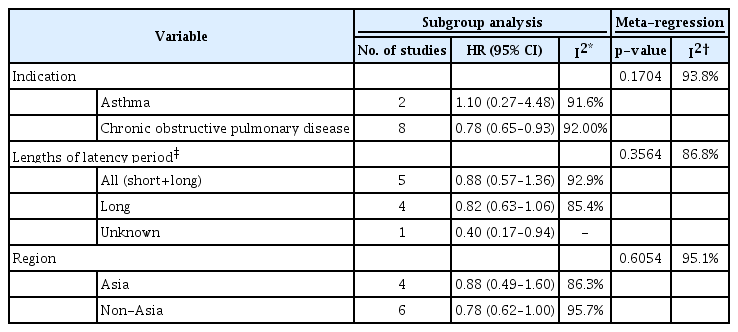
A meta-analysis was performed using the random-effects model, and the analysis was conducted separately by effect measures of HR and OR. Heterogeneity across the studies was assessed with the I2 statistic, and a meta-regression explored sources of heterogeneity. For the HR, which was reported in 10 publications, subgroup analyses were carried out according to indication (asthma vs. COPD), and lengths of the latency period to observe outcome (short- vs. long-term period), and region of studies (Asia vs. non-Asia). The latency period was dichotomized as a short-term period shorter than 1 year versus a long-term period of 1 year or longer in studies that considered the latency between ICS exposure and lung cancer occurrence. In addition, the impact of ICS dose was examined by dichotomizing the dose into low-dose versus high-dose in studies that reported lung cancer occurrence depending upon the ICS dose.
Publication bias was examined using funnel plots and Egger’s test, and the analysis was done using metafor packages in R version 4.1.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
1. Literature search
Our search initially identified 4,058 publications. Of these references, 13 studies were finally included in our analysis (Figure 1). Among them, 10 studies reported HRs for COPD and asthma depending on ICS exposure, whereas the other three studies reported ORs.
2. Baseline study characteristics
The median number and age of the study population in the included studies were 13,686 (range, 712 to 63,276) and 64 years (range, 41 to 72), respectively (Table 1). Nine of the studies were cohort studies, and the remaining four were nested case-control studies. Seven and six studies were conducted in Western and Eastern countries, respectively. The study population was recruited mostly between the 1990s and the 2000s in a retrospective manner. Nine studies were based on national or provincial administrative data, three on multiple hospitals, and one on a sample cohort of national administrative data. Four Taiwanese studies used the same data source, with different recruitment periods and eligibility criteria. The median male proportion of the study population was 47%; however, it varied widely from 0% to 97% across the studies. Most studies could not obtain the smoking history of the study population, and even in studies with available smoking information, the proportion of current smokers was heterogeneous across studies. Four of 13 studies were regarded as high-quality studies based on the Newcastle-Ottawa Quality Scale, whereas the other showed low quality (Supplementary Table S1).
3. Meta-analysis
The pooled HR for developing lung cancer in ICS exposure from 10 studies was 0.81 (95% confidence interval [CI], 0.64 to 1.02; I2=95.7%) (Figure 2). The pooled OR from three studies was 1.02 (95% CI, 0.50 to 2.07; I2=94.7%) (Figure 3). The pooled HR for low-dose ICS was 0.91 (95% CI, 0.85 to 0.98) (Figure 2), and the results were not significantly different between the three studies (I2=0.0%), but for high-dose ICS, the pooled HR was 0.67 (95% CI, 0.30 to 1.54) and the results were significantly heterogeneous between the three studies (I2=82.6%).
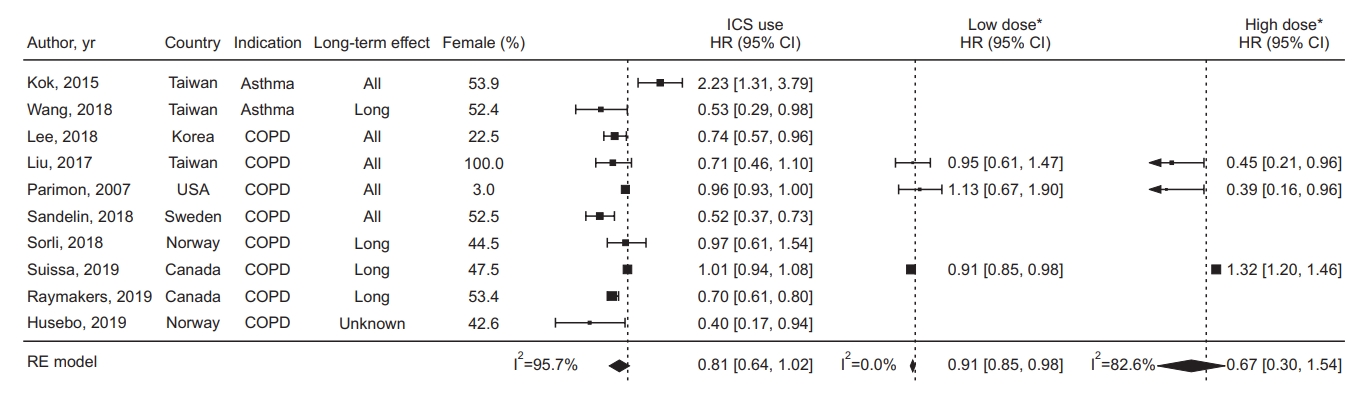
Forest plot in studies assessing the hazard ratio (HR) of inhaled corticosteroid (ICS) exposure. *The threshold for dichotomizing as low-dose versus high-dose was 500 μg fluticasone equivalents. CI: confidence interval; COPD: chronic obstructive pulmonary disease; RE: random effect.
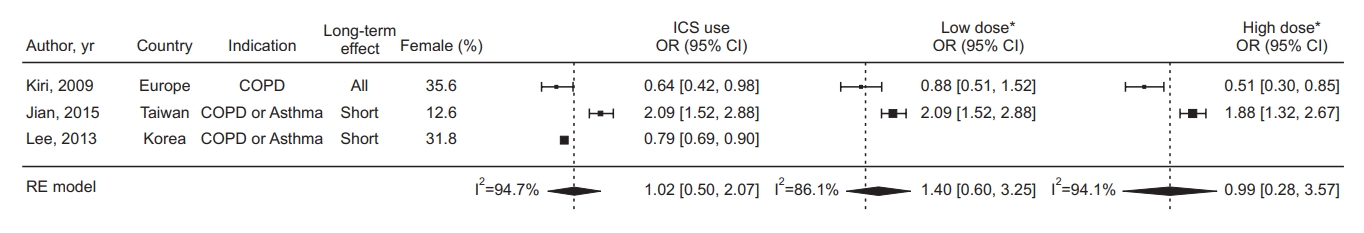
Forest plot in studies assessing the odds ratio (OR) of inhaled corticosteroid (ICS) exposure. *The threshold for dichotomizing as low-dose versus high-dose was 500 ug fluticasone equivalents. CI: confidence interval; COPD: chronic obstructive pulmonary disease; RE: random effect.
Subgroup analyses (Table 2, Figure 4) showed that the estimated pooled HR from two asthma studies was 1.10 (95% CI, 0.27 to 4.48), and the heterogeneity between the two studies was considerable, with I2=91.6%. The pooled HR from eight COPD studies was 0.78 (95% CI, 0.65 to 0.93), and the heterogeneity between the studies was also substantial, with I2=92.0%. Meanwhile, as a result of meta-regression evaluating the difference in HR according to the indication subgroup (Table 2), there was no statistically significant difference as p=0.1704, and the heterogeneity was not unexplained by indications. There was also no statistically significant difference in the analysis results according to the outcome interval or the study region.
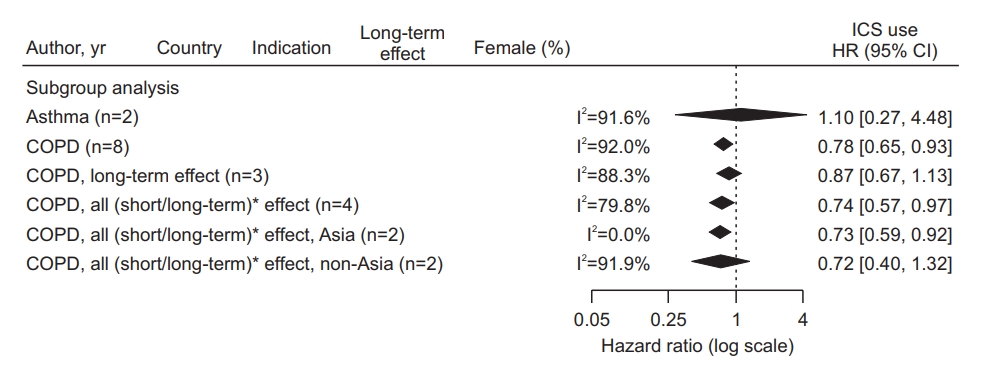
Forest plot of subgroup analysis according to inhaled corticosteroid (ICS) indication, outcome interval, and continents. *The latency period was dichotomized as a short-term period shorter than 1 year versus a long-term period 1 year longer in studies that considered the latency between ICS exposure and lung cancer occurrence. HR: hazard ratio; CI: confidence interval; COPD: chronic obstructive pulmonary disease.
There was no obvious publication bias based on the funnel plot (Supplementary Figure S1) and Egger test (p=0.4777) in the studies assessing the HR of ICS exposure.
4. Eligibility criteria
Of the 13 studies, eight studies targeted COPD and two studies targeted asthma exclusively, whereas the others targeted both COPD and asthma (Table 3). The eligibility criteria for the study population varied across the studies. Some of the studies applied a minimum age varying from 20 to 50 years, while others did not. Most of the included studies enrolled patients with a new diagnosis or new ICS users, but spirometry was used in only two of the studies to include patients with targeted lung disease. Therefore, prescription-based, International Classification of Diseases (ICD) diagnosis code-based, physician-driven, or patient-alleged diagnoses have been heterogeneously applied. The minimum requisite number for diagnosis or prescription also varied from once to thrice during a varying pre-enrollment or study period from 3 months to 1 year. The exclusion criteria mainly included patients with previous lung or any cancers, former ICS use, asthma, and a short follow-up period. Patients with previous lung or any cancers were consistently excluded from the included studies, but those with former ICS use, asthma, and a short follow-up period were handled heterogeneously.
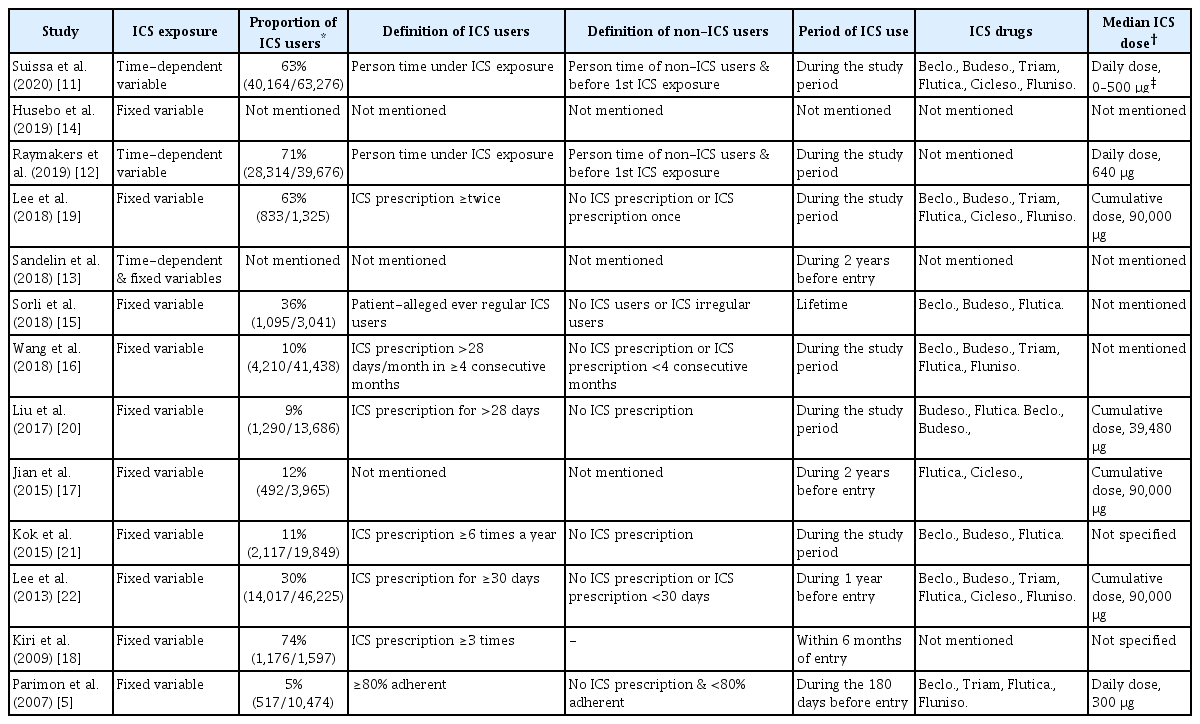
5. Exposure assessment
Three studies [11-13] regarded ICS exposure as a time-dependent variable, whereas the other 10 studies [5,14-22] regarded ICS exposure as a fixed variable (Table 4). The median proportion of ICS users was 30% ranging from 5% to 71%. The 10 studies applied different frequencies and durations for defining ICS users. Controls were never-ICS users in some studies [20,21], while they were both irregular ICS users and never-ICS users in other studies [5,15,16,19,22]. Regarding the period of ICS use, some studies assessed ICS use for the entire study period, whereas others assessed ICS use for 6 months to 2 years within or before study entry. The top three ICS drugs for assessing ICS use were beclomethasone, budesonide, and fluticasone; however, other ICS drugs were also included. Some of the studies provided the median daily or cumulative dose of fluticasone equivalents, which were approximately 500 µg and 39,480–90,000 µg, respectively.
6. Outcome assessment
The median cancer incidence and follow-up periods were 4% and 3.9 years, respectively, although five studies did not provide a median follow-up period of the study population (Table 5). Among ICS users compared to controls, 12 studies assessed the hazard risk for lung cancer development, and two studies assessed the OR. Nine studies found an association between ICS use and reduced development of lung cancer, whereas four studies did not find such an association. Adjusted confounders were heterogeneous across the studies, and smoking was adjusted in four of the 13 studies. Only four studies introduced 1 to 2 latency years in their main or subgroup analysis, assuming that ICS can be biologically effective in preventing lung cancer development at least 1 to 2 years before the establishment of a lung cancer diagnosis. In addition, only four studies avoided immortal time bias by not allocating the unexposed period to ICS as the exposure period.
Discussion
This critical systematic review highlighted the extreme heterogeneity of studies on the protective effects of ICS against lung cancer development. We provisionally meta-analyzed HRs and ORs for developing lung cancer in ICS exposure: the pooled HR, 0.81 (95% CI, 0.64 to 1.02; I2=95.7%); the pooled OR, 1.02 (95% CI, 0.50 to 2.07; I2=94.7%). The protective effect of ICS was inconsistent between HR and OR, and the pooled HR and OR both had substantial heterogeneity that potential sources of heterogeneities could not explain on meta-regression. This heterogeneity cannot be handled beyond mathematical pooling using a random-effects model, as heterogeneity exists in all domains, including study characteristics, eligibility criteria, exposures, and outcome assessments. Further, we would like to discuss how these heterogeneities were observed in the studies included in the analysis and how they can affect the study outcome.
First, the eligibility criteria for selecting the study population varied across studies. In addition, smoking is the most influential carcinogen for the development of lung cancer [23] but was not adjusted in a considerable proportion of the studies [11-13,15,17,20,22]. Lung cancer generally develops after 40 years; however, five studies included patients aged <40 years [15,17,19,21,22]. In particular, it was different for each study whether it included only newly diagnosed airway diseases/new ICS users and whether it enrolled all patients with airway diseases using ICS without any restrictions. Assuming that the duration of the disease and the period of exposure to the medication affect the incidence of lung cancer, the effects may be underestimated or overestimated if the degree of exposure is not accurately measured. Therefore, to obtain proper outcomes, the treatment duration from personal first ICS should be defined as exposure only for patients who were diagnosed with airway diseases for the first time.
Second, the studies assessed the degree of ICS exposure differently. Some studies assessed ICS exposure during the entire study period, whereas others assessed ICS exposure in a limited time window before or during the study period. While there have been studies that simply used the absolute dose as the criterion for ICS use, there have also been studies that applied weights for the duration of ICS use. In principle, the ICS dose with weights for the duration could quantify ICS exposure to each patient more accurately than the absolute dose. In addition, the confirmation of actual ICS use showed various aspects. Most studies identified the regular use of ICS, but some studies only checked prescription records, which can affect the results of the analysis.
Finally, in terms of the outcome measure, the latency period from the initiation of observation is varyingly applied across studies. Some studies have placed a latency period of 1 to 2 years, while other lack this period. Considering the process of lung cancer development, it is challenging to prove the causality of the occurrence of lung cancer immediately after a short exposure period. Therefore, ensuring a minimum latency period is essential. There were significant differences in the incidence rate of lung cancer between the studies, which could be biased. In addition, it was observed that the highest risk factor was not properly controlled because smoking history was not included as an adjusted confounder due to the methodological limitations of the study.
The mechanism of action of ICS in the development of lung cancer has not been clearly described. Although chronic airway inflammatory diseases, including COPD and lung cancer, affect the lungs distinctly, airflow obstruction in COPD has been reported as a risk factor for lung cancer independent of smoking [24-28]. Both programmed aging and non-programmed death are presumed to be key common mechanisms in developing lung cancer and COPD [29-31]. Several genetic factors have been reported to be commonly involved in COPD and lung cancer [32-34]. From the acquired perspective, the hypothesis that specific inflammatory microenvironments expressed in chronic airway diseases form the lung cancer-prone condition is the most convincing. Specifically, the Th1 inflammatory microenvironment promotes the generation of reactive oxygen species [35] and activates transcription factors such as nuclear factor kappa B (NF-κB) and activator protein 1 (AP-1) [36], leading to carcinogenesis. In addition, the increased secretion of myeloperoxidase (MPO), neutrophil elastase, and matric metallopeptidase 9 (MMP9) induced by interleukin 17 provides an environment for promoting tumor growth [37,38]. Although ICS has a major effect on controlling Th2 inflammation, it can be estimated that the role of ICS in regulating the inflammatory process may inhibit carcinogenesis.
In conclusion, studies published to date on ICS and lung cancer incidence had heterogeneous study populations, study designs, exposure definitions, and outcome assessments to generate a pooled conclusion. Understanding these heterogeneities will help future researchers establish robust evidence of ICS and lung cancer incidence.
Notes
Authors’ Contributions
Conceptualization: Lee SY, Yoon SH. Methodology: Lee SY, Yoon SH, Hong H. Formal analysis: Lee SY, Yoon SH, Hong H. Data curation: Lee SY, Yoon SH. Project administration: Lee SY. Resources: Lee SY. Software: Lee SY, Hong H. Supervision: Lee SY. Validation: Lee SY, Yoon SH, Hong H. Visualization: Lee SY, Yoon SH. Investigation: Lee SY, Yoon SH, Hong H. Writing - original draft preparation: Lee SY, Yoon SH, Hong H. Writing - review and editing: Lee SY, Yoon SH, Hong H. Approval of final manuscript: all authors.
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Funding
No funding to declare.
Supplementary Material
Supplementary material can be found in the journal homepage (http://www.e-trd.org).
Quality assessment of the included studies.
Funnel plots for studies assessinging the hazard ratio (HR) of inhaled corticosteroid exposure.