Summary of Korean Asthma Guideline
Article information
Abstract
Asthma is a chronic inflammatory airway disease that is characterized by variable airflow obstruction. The Korean Asthma Study Group of the Korean Academy of Tuberculosis and Respiratory Diseases has recently updated the Korean Asthma Guideline. This review summarizes the updated Korean Asthma Guideline. Asthma prevalence is increasing worldwide, and in Korea. Variable airflow obstruction can be confirmed by bronchodilator response or other tests, and should be established prior to the controller medication. A low-dose inhaled corticosteroid-formoterol is used to alleviate symptoms in all treatment step, and it can be used as a controller as well as reliever in steps 3–5. This approach is preferred, because it reduces the risk of severe exacerbations, compared to the use of short-acting β2-agonist as reliever. In severe asthma, phenotype/endotype based on the underlying inflammation should be evaluated. For type 2 severe asthma, the biologics should be considered.
Introduction
Asthma is a chronic inflammatory airway disease that is characterized by variable airflow obstruction [1]. The Korean Asthma Study Group is a study group of the Korean Academy of Tuberculosis and Respiratory Diseases (KATRD). The Korean Asthma Guideline by KATRD was first published in 2000. The Korean Asthma Guideline was updated in 2005, 2014, and 2020 [2]. The Korean Asthma Study Group has further updated the Korean Asthma Guideline in 2022.
One of the main reasons for publishing the 5th revised edition is that several very important studies have been published in the last 2 to 3 years, especially in the field of asthma treatment. The treatment paradigm of patients with mild asthma has changed. In addition, many recent landmark studies on new biologics have been published. To reflect recent trends in global asthma treatment, the Korean Asthma Study Group organized the committee and published the updated guideline.
This 5th revision is based on the contents of the 4th revision in 2020. Also, this guideline adopts the 2021 Global Initiative for Asthma Strategy Report [3]. Based on these two documents, the latest knowledge in asthma has been updated through a systematic review. The results of research conducted on Korean patients are also reflected. The severe asthma section is newly developed in a separate chapter. An evaluation and management algorithm for severe asthma is created. This review summarizes the updated Korean Asthma Guideline.
Definition, Epidemiology, and Burden of Asthma
Asthma is characterized by respiratory symptoms, such as wheezing, dyspnea, chest tightness, and cough with variable expiratory airflow limitation. Asthma prevalence is increasing worldwide, and in Korea. Many asthma patients still die due to inadequate management. Asthma is a chronic disease that consumes substantial medical resources, and its prevalence is very high, causing a significant economic burden on individuals and society. The prevalence of asthma in Korea is about 3.2% to 4.7%, and it is increasing, mainly in children and the elderly. The rate of pulmonary function tests in asthma patients is low in Korea. The rate of prescription of inhaled corticosteroid (ICS) is low, while oral medications are high [4].
The Diagnosis and Assessment of Asthma
Asthma is diagnosed by characteristic symptoms and variable expiratory airflow obstruction. Variable airflow obstruction can be confirmed by bronchodilator response or other tests, and should be established prior to the controller medication. In the diagnosis stage of asthma, triggering factors, comorbidities, patient knowledge, device technique, and severity should be assessed. The severity of asthma can change over time. It can be assessed not only by the severity of the underlying disease, but also by the level of treatment and response to the treatment. It is important to differentiate between severe asthma and uncontrolled asthma. In addition to control status, device technique, compliance, side effects, comorbidities, and future risks should be evaluated. Well control can be defined when there are less than three times/week daytime symptoms and no activity limitation, no nocturnal asthma symptoms, less than three times/week reliever medication use, and normal lung function [3]. Along with symptom control, risk factors for future exacerbations (greater or equal to one severe exacerbation in previous year, poor compliance, incorrect inhaler technique, low lung function, smoking, eosinophilia, fixed airflow limitation, and adverse drug reactions) should be assessed. The most useful indicator of future risk is lung function [5-8]. Pulmonary function test should be performed at diagnosis, 3 to 6 months after treatment, and periodically thereafter. If there is a discrepancy between symptoms and lung function, additional evaluations are needed.
A patient may have both asthma and chronic obstructive pulmonary disease (COPD) together, which can be defined as asthma COPD overlap (ACO) [9]. ICS combined with bronchodilator is recommended in patients with ACO. Differential diagnosis of asthma includes COPD, vocal cord dysfunction, bronchiectasis, chronic eosinophilic pneumonia, and eosinophilic bronchitis. Asthmatic patients may have Churg-Strauss syndrome or allergic bronchopulmonary aspergillosis.
Treatment of Asthma
Controller treatment based on ICS should be started as soon as possible after the diagnosis of asthma, and the initial controller therapy can be selected according to the frequency and severity of the patient’s symptoms (Figure 1) [3]. The recommended treatment options are as follow: When the patient has infrequent symptoms (less than twice a month) and no risk factors for exacerbations, the preferred initial treatment is as needed low-dose ICS-formoterol. When the patient has asthma symptoms or needs a reliever twice a month or more, the preferred initial treatment is also as needed low-dose ICS-formoterol. When the patient has troublesome asthma symptoms most days, or wakes due to asthma once a week or more, the preferred initial treatment is low-dose ICS-formoterol maintenance and reliever therapy (especially if any risk factors for exacerbations exist). When the initial asthma presentation is with severely uncontrolled asthma, or with an acute exacerbation, the preferred initial treatment is medium dose ICS-formoterol maintenance and reliever therapy. In this case, a short course of oral corticosteroids (OCSs) may also be needed.
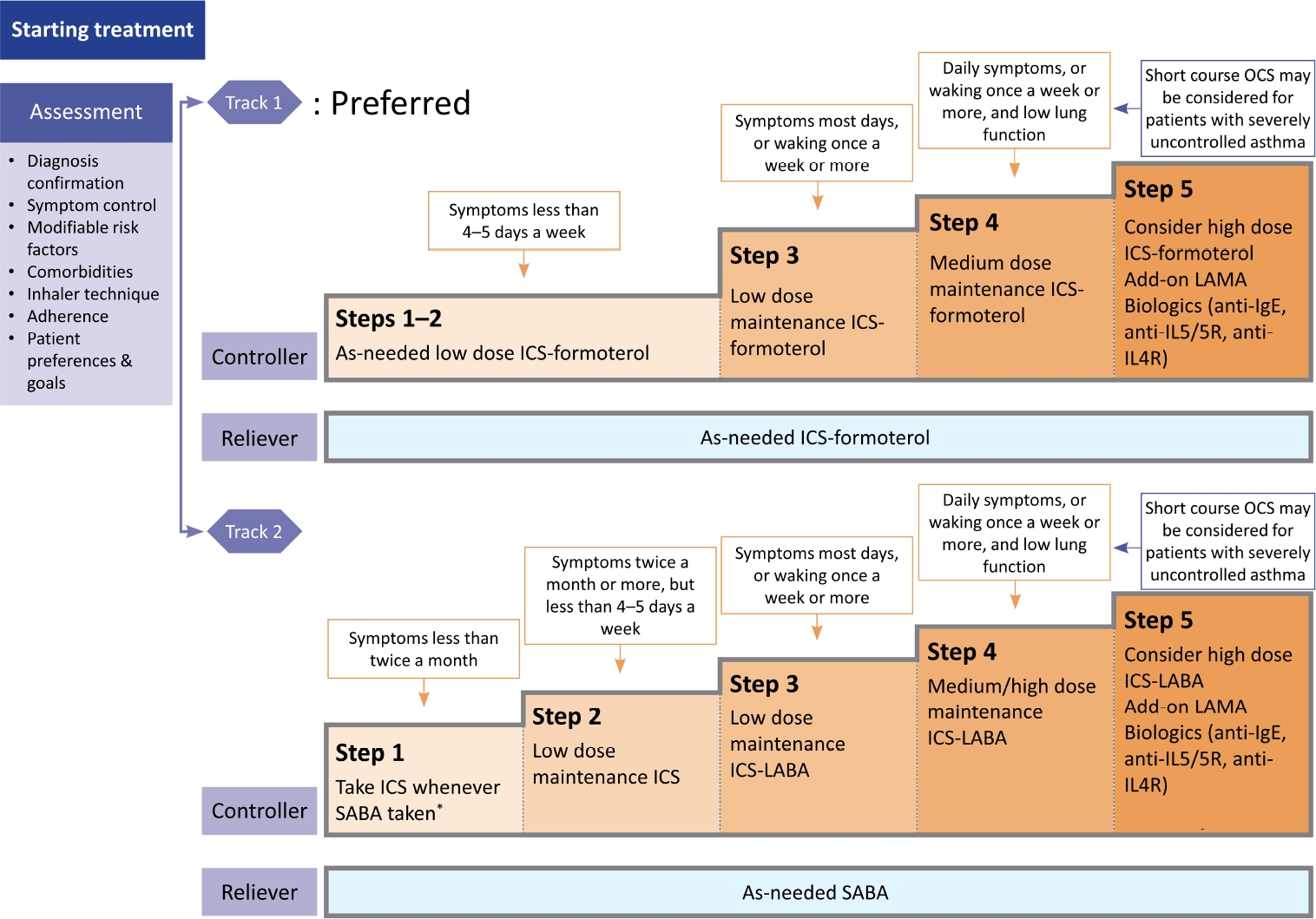
Selection of initial medication in patients with stable asthma. Modified from Reddel et al [3]. *Evidence for taking inhaled corticosteroid (ICS) whenever short-acting β2-agonist (SABA) taken is limited. Taking ICS whenever SABA is taken is off-label in Korea. OCS: oral corticosteroid; LAMA: long-acting muscarinic antagonist; IgE: immunoglobulin E; IL5: interleukin-5; IL4R: interleukin-4 receptor; LABA: long-acting β2-agonist.
Treatment Stage
1. Track 1: Use a low-dose ICS-formoterol as reliever
A low-dose ICS-formoterol is used to alleviate symptoms in all treatment stages, and it can be used as a controller as well as reliever in stages 3–5. This approach is preferred, because it reduces the risk of severe exacerbations, compared to the use of short-acting β2-agonist (SABA) as reliever [10-14].
2. Track 2: Use a SABA as reliever
This track can be applied as an alternative when it is difficult to follow track 1. In step 1, a low-dose ICS should be administered together when using a SABA.
3. Preferred treatment for each stage
• Steps 1 and 2: Use a low-dose ICS-formoterol as needed. Alternatively, a SABA can be used as needed while using a low-dose ICS every day. However, there is a risk of SABA treatment alone due to low compliance with ICS maintenance treatment in patients with mild asthma.
• Step 3: Use a low-dose ICS-formoterol maintenance and reliever therapy. If acute exacerbation persists, maintenance of a low-dose ICS-formoterol (budesonide–formoterol or beclometasone–formoterol) is recommended. This strategy is more effective to prevent exacerbation, compared with the same dose of ICS plus long-acting β2-agonists (LABAs) as controller and SABA as reliever [15-18].
• Step 4: Use a medium dose ICS-formoterol maintenance and reliever therapy. Before escalating the ICS dose, poor device technique, low compliance, environmental exposure, and whether the patient’s symptom is due to asthma should be evaluated.
• Step 5: Consider referral to an asthma specialist. Evaluation of phenotype/endotype and biologics is recommended.
Once asthma treatment is initiated, periodic evaluation, adjustment of treatment, and review of treatment response are necessary. In each patient, modifiable risk factors should be managed. The controller can be increased or decreased step-by-step to control symptoms and minimize exacerbation, airflow limitation, and the side effects of drugs. Once asthma is well controlled for 2 to 3 months, treatment should be stepped down to find the lowest ICS dose.
Management of Acute Exacerbation
Asthma exacerbation can be defined as an acute or subacute worsening of a patient’s symptoms or lung function than usual. In some patients, acute exacerbation can be a first manifestation of asthma. Risk factors that increase asthma-related death should be identified. To assess the severity of exacerbation of asthma, history-taking and physical examination should be performed simultaneously with the initiation of treatment.
When there is a sign of exacerbation, inhaled β2-agonist, oxygen, and systemic steroid should be administered. In a primary care clinic, when exacerbation is severe, immediate transfer to the emergency treatment facility should be considered. For mild and moderate asthma exacerbations, repeated inhalation of SABA (up to 4 to 10 puffs every 20 minutes for the first hour) is an effective initial treatment.
Early administration of systemic steroid is recommended. Systemic steroid reduced mortality, relapse, hospitalization, and the use of reliver [19]. Severe asthma exacerbation is a life-threatening condition, and requires immediate and effective treatment. Symptoms, oxygen saturation, and lung function should be re-evaluated after treatment.
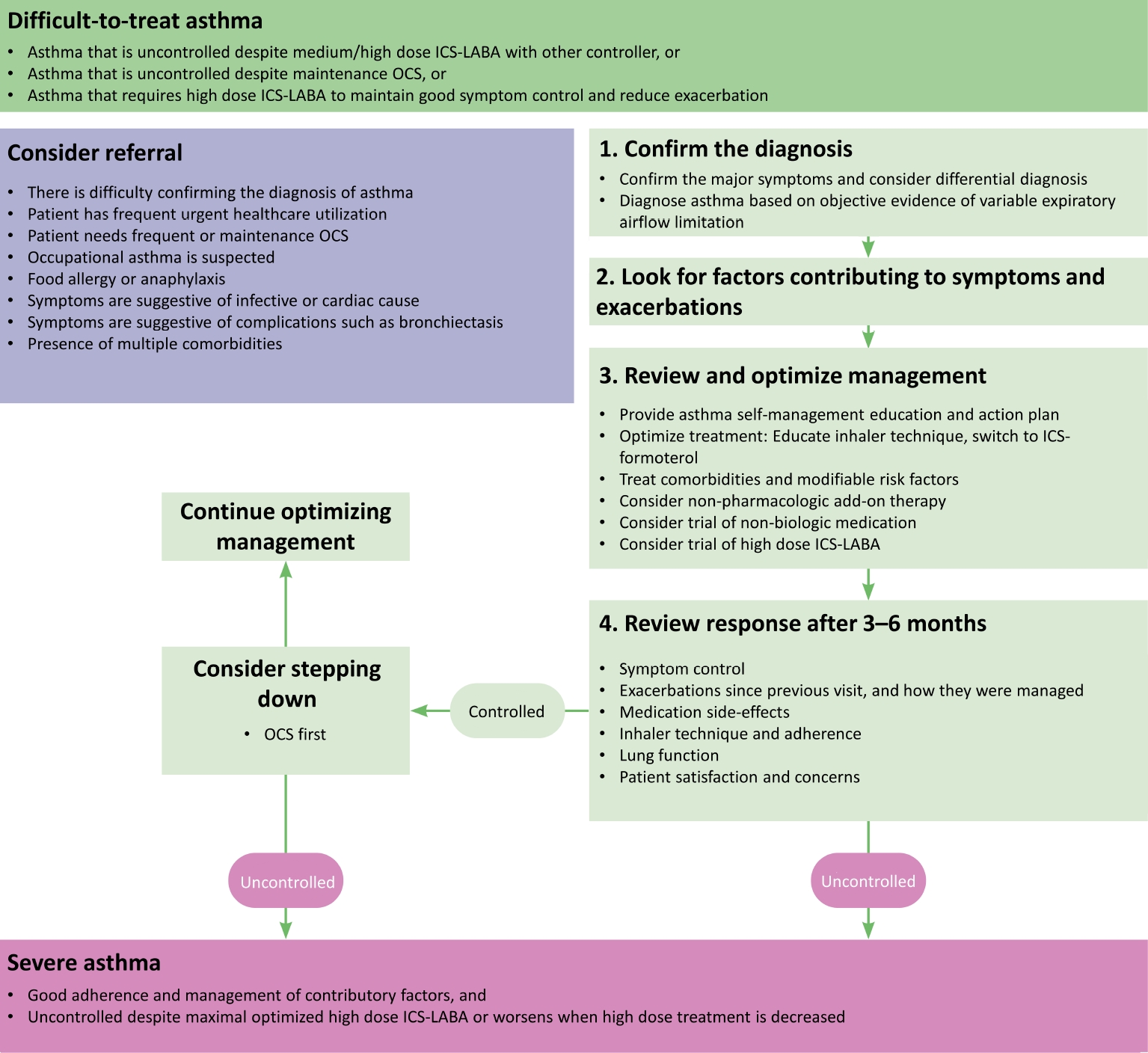
Severe Asthma
Difficult-to-treat asthma is a condition that is not controlled despite the use of moderate or high-dose ICS and other controllers, or where the maintenance of OCS is needed. Among them, severe asthma can be defined when the patient has good adherence, all factors contributing to the worsening of asthma symptoms are being treated, and asthma is not controlled despite the optimized use of high-dose ICS plus LABA, or worsening when the treatment dose is reduced (Figure 2). Phenotype/endotype based on the underlying inflammation should be evaluated, and additional medication should be provided accordingly (Figure 3). For type 2 severe asthma, biologics should be considered. When a biological agent is added, the response should be evaluated 3 to 4 months after the start of administration, and every 3 to 6 months thereafter to determine whether to continue.
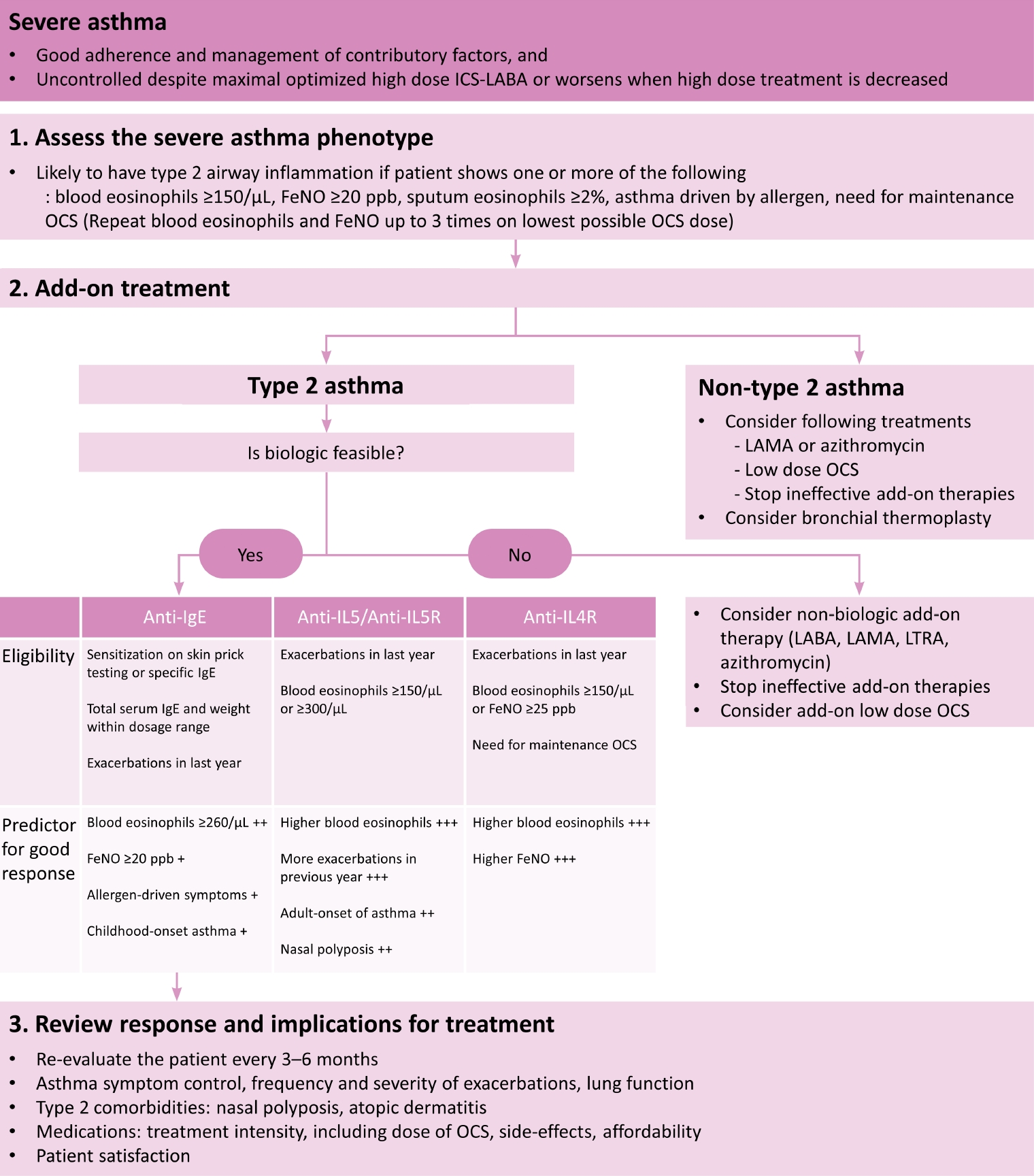
Algorithms of assessment and management in patients with severe asthma. Modified from Reddel et al [3]. ICS: inhaled corticosteroid; LABA: long-acting β2-agonist; FeNO: fractional exhaled nitric oxide; OCS: oral corticosteroid; LAMA: long-acting muscarinic antagonist; IgE: immunoglobulin E; IL5: interleukin-5; IL5R: interleukin-5 receptor; IL4R: interleukin-4 receptor; LTRA: leukotriene receptor antagonist.
Asthma in Specific Populations or Settings
Cough variant asthma is a subtype of asthma whose main symptom is chronic cough. It is diagnosed by confirming airway hyperresponsiveness, and treatment is based on ICS. Exercise-induced bronchoconstriction (EIB) characteristically occurs immediately after exercise, and resolves within 30 minutes. Regular ICS is recommended for patients with EIB.
Occupational history should be taken in patients with newly diagnosed asthma. It is mandatory to prevent exposure to allergens or other sensitizing agents at work. Early diagnosis and intervention are important.
The clinical course of aspirin-exacerbated respiratory disease (AERD) is well established [20]. It begins with rhinitis symptoms, and progresses to chronic rhinosinusitis with nasal polyps. Asthma develops subsequently. AERD can be diagnosed by aspirin challenge test. Aspirin or nonsteroidal anti-inflammatory drug should be avoided. ICS is the main treatment of asthma in AERD. Aspirin desensitization treatment can significantly improve the quality of life. In particular, aspirin desensitization reduces the recurrence of nasal polyps and OCS use [21].
To prevent surgical complications related to asthma, it is necessary to accurately evaluate the asthma control status before surgery. Preventive treatment before surgery can decrease the risk of post-operative complications in patients with asthma.
The benefit of treating asthma during pregnancy outweigh the potential side effects of asthma medication on the fetus [22]. Poorly controlled asthma is associated with worse outcomes for the baby [22]. Most asthma medications, including ICS plus LABA, were not associated with increased incidence of fetal abnormalities [23]. ICS reduces the risk of asthma exacerbation during pregnancy [24,25].
Weight loss can improve asthma control in obese patients [26,27]. The main treatment for obese asthma patients is ICS, and weight loss should be included in the treatment. Rhinitis often precedes asthma, and is a risk factor for asthma [28]. Treatment of rhinitis can improve asthma symptom [29,30].
Notes
Authors’ Contributions
Conceptualization: Rhee CK, Lim SY, Yoon HK, Lee SY. Methodology: Rhee CK, Moon JY, Joo H, Jung JY, Lee JK, Min KH, Koo HK. Formal analysis: Rhee CK. Data curation: Rhee CK, Moon JY, Joo H, Jung JY, Lee JK, Min KH, Koo HK. Software: Rhee CK. Validation: Rhee CK. Investigation: Rhee CK, Moon JY, Joo H, Jung JY, Lee JK, Min KH, Koo HK. Writing - original draft preparation: Rhee CK. Writing - review and editing: Rhee CK, Moon JY, Joo H, Jung JY, Lee JK, Min KH, Koo HK, Lim SY, Yoon HK, Lee SY. Approval of final manuscript: all authors.
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Funding
No funding to declare.