Progressive Pulmonary Fibrosis: Where Are We Now?
Article information
Abstract
Interstitial lung diseases (ILDs) are a diverse collection of lung disorders sharing similar features, such as inflammation and fibrosis. The diagnosis and management of ILD require a multidisciplinary approach using clinical, radiological, and pathological evaluation. Progressive pulmonary fibrosis (PPF) is a distinct form of progressive and fibrotic disease, occurring in ILD cases other than in idiopathic pulmonary fibrosis (IPF). It is defined based on clinical symptoms, lung function, and chest imaging, regardless of the underlying condition. The progression to PPF must be monitored through a combination of pulmonary function tests (forced vital capacity [FVC] and diffusing capacity of the lung for carbon monoxide), an assessment of symptoms, and computed tomography scans, with regular follow-up. Although the precise mechanisms of PPF remain unclear, there is evidence of shared pathogenetic mechanisms with IPF, contributing to similar disease behavior and worse prognosis compared to non-PPF ILD. Pharmacological treatment of PPF includes immunomodulatory agents to reduce inflammation and the use of antifibrotics to target progressive fibrosis. Nintedanib, a known antifibrotic agent, was found to be effective in slowing IPF progression and reducing the annual rate of decline in FVC among patients with PPF compared to placebos. Nonpharmacological treatment, including pulmonary rehabilitation, supplemental oxygen therapy, and vaccination, also play important roles in the management of PPF, leading to comprehensive care for patients with ILD. Although there is currently no cure for PPF, there are treatments that can help slow the progression of the disease and improve quality of life.
Introduction
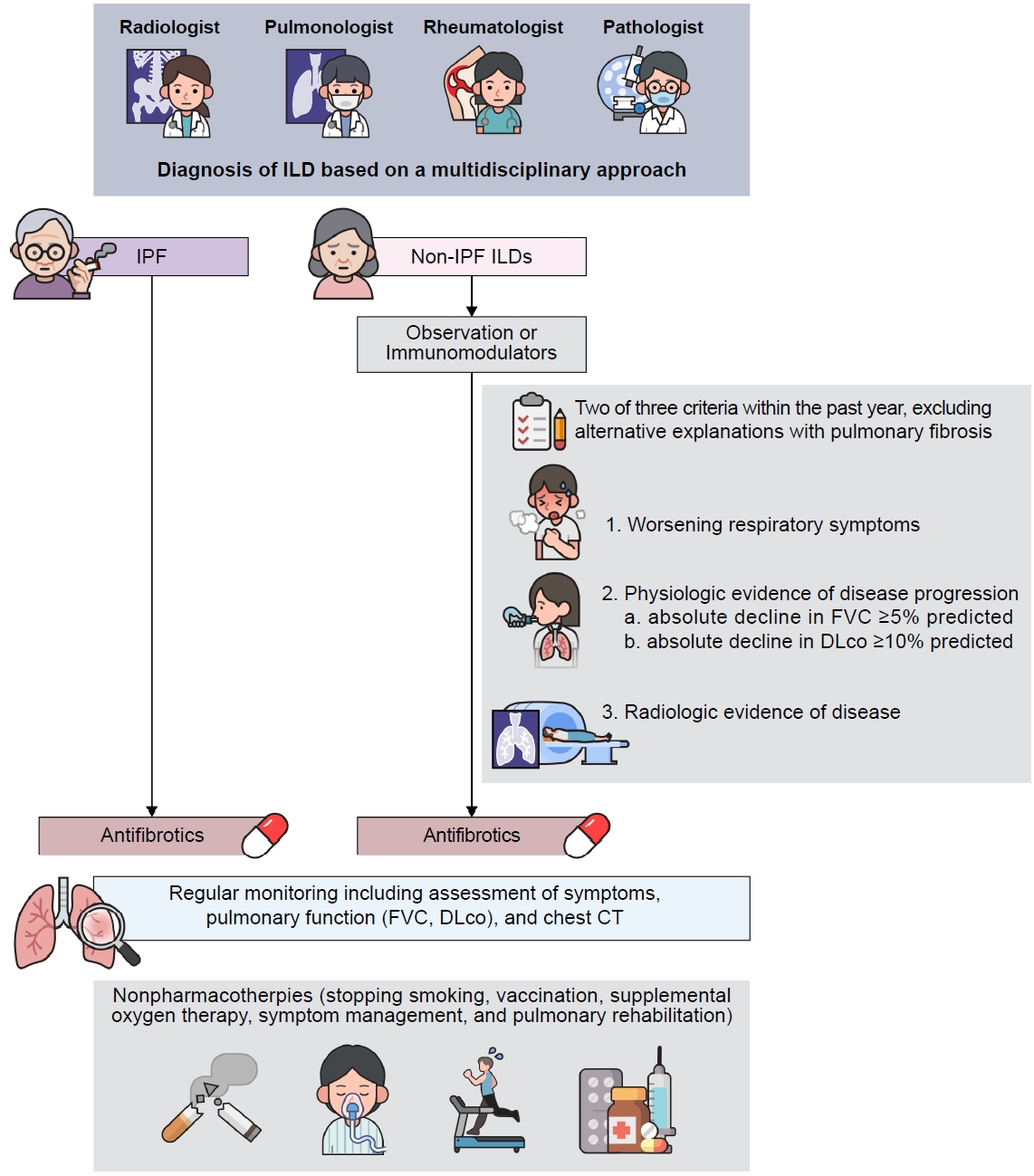
Interstitial lung disease (ILD) is a group of >200 heterogenous lung disorders that are classified together based on shared clinical, radiographic, and pathological features [1-3]. Despite the heterogeneity of this group of diseases, they also show commonalities in terms of pathophysiology, including inflammation and fibrosis in the lung parenchyma [4]. These disorders typically present with progressive dyspnea, a restrictive abnormality with impaired diffusing capacity on physiologic testing, and diffuse bilateral infiltrates on chest imaging [1]. The diagnostic and management decisions of ILD are based on a multidisciplinary approach that includes clinical, radiographical, and pathological findings [2,5].
Idiopathic pulmonary fibrosis (IPF) is a chronic progressive fibrosing ILD (PF-ILD) of unknown cause that occurs primarily in older adults [6] and is characterized by a usual interstitial pneumonia (UIP) pattern on chest computed tomography (CT) or histopathology [6]. The natural history of IPF usually shows an insidious decline in lung function resulting in progression to respiratory failure and death on average within approximately 3 to 5 years after the initial diagnosis [7]. Compared to IPF, some non-IPF ILDs are managed more effectively with immunosuppressive therapy and have relatively less severe outcomes [5,6]. However, regardless of the initial diagnosis, a number of ILDs other than IPF manifest as progressive pulmonary fibrosis (PPF) with common pathogenetic mechanisms and disease behavior similar to IPF [7,8]. In this review, we aim to summarize the current literature on PPF in various ILDs, with a focus on the diagnostic process, clinical significance, and managements.
Definitions of PPF
Fibrosing ILD refers to a group of lung disorders characterized by the presence of fibrosis or scarring within the lung parenchyma [9]. The diagnosis typically involves a combination of clinical evaluation, imaging tests, pulmonary function tests, and occasionally a lung biopsy [7]. Among ILDs other than IPF, PPF refers to an ILD that exhibits radiological signs of fibrosis and demonstrates evidence of progression over time [7]. Approximately 13% to 40% of non-IPF fibrosing ILDs are expected to progress within 2 years, even if they are managed appropriately [9].
According to the official American Thoracic Society (ATS)/European Respiratory Society (ERS)/Japan Respiratory Society (JRS)/Asociación Latinoamericana de Tórax (ALAT) clinical practice guideline [7], PPF is defined as when at least two of the following three criteria have occurred within the past year with no alternative explanation in patients with ILD of known or unknown etiology other than IPF, which has radiological evidence of pulmonary fibrosis:
(1) Worsening respiratory symptoms
(2) Physiological evidence of disease progression within 1 year of follow-up (either of the following):
a. Absolute decline in forced vital capacity (FVC) ≥5% predicted.
b. Absolute decline in diffusing capacity of lung for carbon monoxide (DLco) (corrected hemoglobin) ≥10% predicted.
(3) Radiological evidence of disease progression (one or more of the following):
a. Increased extent or severity of traction bronchiectasis and bronchiolectasis.
b. New ground-glass opacity with traction bronchiectasis.
c. New fine reticulation.
d. Increased extent or coarseness of reticular abnormality.
e. New or increased honeycombing.
f. Increased lobar volume loss.
Previous studies defined this progressive phenotype ILD using term “PF-ILD” [10-12]. The criteria for assessing progression were not significantly different across studies, but they varied in the timeframe, ranging from 6 months to 2 years, and the evaluation methods also differed slightly, with some focusing solely on pulmonary function and others considering a combination of imaging and clinical symptoms [10-12]. The guideline was based on previous studies to define PPF, and it used the term “PPF” instead of “PF-ILD” because disease progression is the result of PPF beyond the interstitial space in the lung parenchyma, resulting in a clinical course similar IPF [7].
PPF is not a specific disease. Its definition is based on clinical symptoms, lung function, and chest imaging, regardless of the underlying condition. Therefore, when ILDs are diagnosed, the progression to PPF must be monitored through a combination of pulmonary function tests, including a consistent decline in FVC and DLco, assessment of symptoms, and CT scans, with regular follow-up.
Pathogenesis of PPF
The pathogenetic mechanisms of PPF are not yet fully understood, but it has been suggested that some mechanisms involved in IPF could also play a role in the development of progressive fibrosis in non-IPF ILDs [13]. The combination of genetic, environmental, and host factors in IPF can initiate an immunoinflammatory response or lead to the hyperactivation of resident cells, such as epithelial or endothelial cells [13]. Following repetitive injury to alveolar or endothelial cells, as well as immune activation and inflammation, fibroblasts can be activated by profibrotic cytokines [4,13]. This activation triggers their proliferation and differentiation into myofibroblasts, leading to the excessive secretion of extracellular matrix (ECM) proteins and lung remodeling [6,13]. Consequently, antifibrotic drugs may be effective in slowing the decline of lung function in patients with IPF, but antiinflammatory drugs have not been shown to be effective [14,15].
In contrast to IPF, in some patients with non-IPF ILDs, the fibrotic response may be halted spontaneously through endogenous regulatory mechanisms after antigen removal or with immunomodulatory treatment [4,13]. This is because fibrosis in these conditions can be driven by inflammation [13]. However, progressive fibrotic phenotypes are characterized by the accumulation of ECM and lung remodeling, which are maintained by self-progression and activation loops [13]. The UIP pattern can be seen in non-IPF ILDs, such as connective tissue disease (CTD)-associated ILD (CTD-ILD) and hypersensitivity pneumonitis (HP), and is associated with a progressive disease course similar to that of IPF [16].
ILDs Associated with PPF
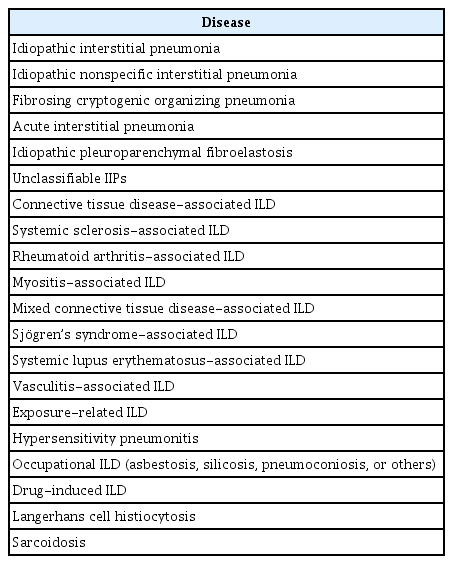
Table 1 summarizes ILDs that can manifest as PPF. Although IPF is a prototype of PF-ILD, it is excluded from the definition of PPF. The reported incidence of various ILD subtype exhibited significant heterogeneity [17]. In a nationwide epidemiologic study for 11,347 non-IPF ILDs demonstrated that 52% were unclassifiable idiopathic interstitial pneumonia (IIP) and 46% were autoimmune disease, with half of the latter having PPF [18].
Idiopathic nonspecific interstitial pneumonia (iNSIP) presents with bilateral ground-glass and irregular reticular opacities with traction bronchiectasis on high-resolution CT [5]. In 32% of patients with iNSIP, this may progress to a UIP-like pattern with honeycombing on chest CT [4,7,16]. Fibrosing cryptogenic organizing pneumonia (COP) is a rare entity with a similar clinical and histological appearance to COP but with variable amounts of fibrosis [19]. Acute interstitial pneumonia is characterized by diffuse interstitial fibrosis, and if it progresses, it can result in the distortion of bronchovascular bundles and traction bronchiectasis [5]. Idiopathic pleuroparenchymal fibroelastosis (iPPFE) is a rare condition that is characterized by fibrosis affecting the pleura and subpleural parenchyma, primarily in the upper lobes, with progression seen in 60% of patients [5,20]. Unclassifiable IIPs, which represent combined or overlapping patterns of IIPs, were associated with disease progression in 40% of patients [21].
ILD can be a feature of several vasculitides and CTDs, including systemic sclerosis (SSc), rheumatoid arthritis (RA), myositis, mixed connective tissue disease (MCTD), Sjögren’s syndrome (SS), and systemic lupus erythematosus (SLE). ILD affects 40% to 50% of patients with CTD [22], in whom the dysregulation of the immune system leads to the release of profibrotic cytokines, chemokines, and growth factors by inflammatory cells. These factors then stimulate fibroblast proliferation and differentiation to myofibroblasts, leading to the formation of fibrosis [13]. The prevalence of PPF in CTD-ILDs was reported to be 32% in SSc-ILD, 40% in RA-ILD, 6% in myositis, 15% in MCTD, 24% in SS-ILD, and 24% in SLE-ILD [4,22]. Pulmonary fibrosis with vasculitis was also reported to progress in 35% of cases [23].
HP is an immunologically mediated lung disease resulting from exposure, and persistent exposure plays a role in the development of fibrosis [13]. Fibrotic HP is known to progress in 21% of patients [4]. Occupational and drug-induced ILDs occur as a result of exposure to certain substances in the workplace or to drugs, leading to inflammation and eventual fibrosis of the lung interstitium [24,25]. Advanced forms of occupational ILDs may have a similar clinical presentation to diffuse interstitial fibrosis [25]. Although drug-induced ILD rarely has the UIP pattern, it may present with progressive fibrosis [4,24].
Langerhans cell histiocytosis (LCH) is a group of disorders characterized by the infiltration of tissues by large numbers of Langerhans cells. Overall, 30% to 40% of patients with LCH show persistent symptoms of variable severity with the conversion of radiological nodules into thick-walled and then thin-walled cysts that remain stable over time [26]. End-stage fibrosis is an uncommon manifestation of LCH, and only a minority of patients with LCH progress to this stage [27]. Sarcoidosis is a granulomatous inflammatory disorder of unknown etiology. Fibrosis can arise from chronic granulomatous inflammation, and there is an estimated occurrence of significant progressive fibrosis in approximately 5% of patients with pulmonary sarcoidosis [7,27].
Risk Factors and Outcomes
Several cohort studies investigating patients with fibrosing ILD have identified risk factors associated with PPF (Table 2) [28-32]. A prospective cohort of 2,746 patients with fibrotic ILD revealed that 47% of 2,028 patients with non-IPF fibrosing ILDs experienced disease progression within 2 years [28]. Age, male sex, current smoking, fibrotic HP, time to diagnosis ≥12 months, dyspnea at the onset of the disease, weight loss, velcro crackles, and lower lung functions (including FVC and DLco) were reported to be risk factors associated with PPF among non-IPF fibrosing ILDs [28,31,32].
Many serum biomarkers have been suggested for monitoring the early identification of PPF. A systematic review and meta-analysis of 43 studies showed that the Krebs von den Lungen 6 levels in progressive ILD group was 325.98 U/mL higher than that in the non-progressive ILD group [33]. Also, a separate systematic review that included 13 studies demonstrated a significant association between peripheral blood monocyte counts and disease progression in ILD patients (hazard ratio [HR], 1.83; 95% confidence interval [CI], 1.40 to 2.39; p<0.001) [34]. Moreover, recent investigations have demonstrated that many of the serum biomarkers, matrix metalloproteinase-7 (MMP-7), chemokine ligand-18 (CCL18), surfactant-protein A (SP-A), surfactant-protein D (SP-D), and interleukin-6 (IL-6), are associated with an increased risk of PPF [35]. However, as validation in prospective studies has not been achieved, recommending these biomarkers for early identification of PPF remains challenging, and they may be recommended following further research in the future.
PPF has a worse prognosis, similar to IPF [29-31]. A retrospective observational cohort study of 397 patients with IPF and 447 with non-IPF ILD showed that both IPF and PPF were independent predictors of transplant-free survival, and there was no difference in transplant-free survival between PPF and IPF (HR, 1.12; 95% CI, 0.85 to 1.48; p=0.42) [29]. Patients with PPF had a 91% and 68% transplant-free survival rate at 1 and 3 years, respectively, which was not significantly different compared with patients with IPF [36]. Additionally, the rate of decline in FVC was similar in patients with PPF and IPF, with a mean annual decline of –192.9 mL/year in patients with PPF and –221.0 mL/year in patients with IPF [37]. Cohort studies showed that older age, male sex, lung function (including FVC and DLco), and a UIP-like pattern on chest imaging were associated with mortality or transplant-free survival in patients with PPF [29-31].
Management
1. Pharmacotherapies
1) Antifibrotics
Antifibrotics have been shown to be beneficial in slowing IPF disease progression in numerous studies, and they have been used as the standard treatment of patients with IPF for several years [38]. Based on the clinical and pathophysiological similarities between IPF and PPF, researchers have investigated the role of these antifibrotics in patients with PPF, which yielded meaningful results.
Nintedanib is a tyrosine kinase inhibitor that targets multiple growth factor receptors, including vascular endothelial growth factor, fibroblast growth factor, and platelet-derived growth factor receptor [6]. In a randomized controlled trial (Nintedanib in Progressive Fibrosing Interstitial Lung Diseases [INBUILD]) involving patients with PPF (n=664), nintedanib significantly reduced the annual rate of decline in FVC in the participants compared to the placebo, with a mean difference of –128.2 mL/year [10]. In the post hoc analysis using data from the whole study period of the INBUILD trial, nintenanib was associated with a reduction in ILD progression (HR, 0.66; 95% CI, 0.53 to 0.83; p=0.0003 [predicted absolute decline in FVC ≥10% or death]) (HR, 0.67; 95% CI, 0.46 to 0.98; p=0.04 [acute exacerbation of ILD or death]) [39]. In addition, the effect of nintedanib on reducing the FVC decline rate was not influenced by immunomodulators, which are a mainstay of treatment for non-IPF ILD [40]. Therefore, according to the ATS guideline, nintedanib is recommended for the treatment of PPF in patients who have failed standard management [7].
Pirfenidone is an antifibrotic that exhibits various antiinflammatory and antifibrotic effects, including inhibition of collagen synthesis, downregulation of transforming growth factor beta, and tumor necrosis factor alpha, and suppression of fibroblast proliferation [6]. In a randomized controlled phase 2 trial of pirfenidone in 253 patients with unclassifiable PPF, the planned statistical model could not be applied to the primary endpoint data, but results for key secondary endpoints showed that 24-week of pirfenidone treatment slows disease progression compared to the placebo in patients with unclassifiable PPF, with a mean difference of 95.3 mL [11]. In a separate randomized controlled phase 2b trial (Restrictive versus Liberal Fluid Therapy for Major Abdominal Surgery [RELIEF]) of pirfenidone in 127 patients with PPF, there was no statistically significant difference in the decline of FVC between the pirfenidone and placebo groups due to the premature study termination [12]. However, in a systematic review of two randomized phase 2 trials, pirfenidone was associated with a statistically significant reduction in the mean changes in FVC, with –100 mL/24 wk [41]. The ATS guideline recommended further research into the efficacy, effectiveness, and safety of pirfenidone in PPF [7].
2) Immunomodulators
Immunomodulators are the cornerstone for treating patients with non-IPF ILDs due to the role of inflammation in the pathogenesis. Excess trigger in a predisposed host can lead to more and prolonged inflammation and abnormal repair response, which can lead to fibrosis [42]. Therefore, in contrast to IPF, immunomodulators may have a role by reducing inflammation and potentially slowing down the progression of the fibrotic response in non-IPF ILD by modulating the immune response.
Classification of ILDs is still essential for guiding the decision of initial treatment. In non-IPF ILDs, immunomodulators such as corticosteroids, mycophenolate mofetil (MMF), azathioprine, methotrexate, cyclophosphamide, and rituximab are commonly used [43]. Corticosteroids are often used as the first-line treatment for ILD because of their antiinflammatory and immunosuppressive effects. Sarcoidosis, COP, nonfibrotic HP, and eosinophilic pneumonia generally exhibit favorable responses to corticosteroid treatment [44-46]. A retrospective study of fibrotic HP in 144 patients with the majority of patients having ILD without extensive fibrosis demonstrated that corticosteroids improved survival and slowed fibrotic progression [47]. Some studies have also shown that the combination of corticosteroids with other agents can lead to improvements in pulmonary functions among patients with SSc-ILD [48,49].
MMF is considered the most widely used first-line steroid-sparing agent for fibrosing ILD because it is generally effective, well-tolerated, and less toxic than cyclophosphamide. A cohort study of patients with CTD-ILD showed that MMF was associated with stable or improved lung function over a median follow-up period of 2.5 years [50]. In patients with SSc-ILD, although both MMF and cyclophosphamide treatments resulted in significant improvements in lung function, MMF is preferred because of its better tolerability and lower toxicity [51]. In patients with fibrotic HP, patients treated with MMF or azathioprine also had a significant improvement in DLco and reduced prednisone requirement [52].
Azathioprine has demonstrated effective stabilization or improvement in CTD-ILD, including SSc-ILD [53,54]. Additionally, in sarcoidosis, azathioprine is often used as the second-line therapy [43]. Furthermore, a retrospective study in patients treated with azathioprine showed significant improvement in FVC for fibrotic HP [55]. Methotrexate is an effective second-line therapy as steroid-sparing agents in sarcoidosis [56]. Cyclophosphamide may have a beneficial effect for patients who have rapidly progressive disease or have progressed despite corticosteroids. It is also considered a second-line immunomodulator in CTD-ILD and iNSIP [51,57]. Rituximab has been shown to be effective in progressive CTD-ILD [58,59]. A randomized controlled phase 3 trial (Rituximab and mycophenolate mofetil combination in patients with interstitial lung disease [EVER-ILD]) in patients with CTD-ILD or IIP and a nonspecific interstitial pneumonia (NSIP) pattern showed that combination of rituximab and MMF was superior to MMP alone [60]. Moreover, rituximab is effective as a rescue therapy for fibrosing ILD that is refractory to treatment, and ongoing trials are exploring its potential in severe and progressive NSIP [43,61].
The humanized monoclonal antibody tocilizumab, used for the treatment of early scleroderma, demonstrated a slower decline in FVC over 48 weeks compared to the control group in two randomized controlled trials [62,63]. A retrospective study also demonstrated that tocilizumab has a potential positive effect on stabilizing lung involvement in patients with RA-ILD [64]. In addition, other immunomodulators that may have biologic activity against ILDs include tofacitinib, leflunomide, abatacept, tacrolimus, and intravenous immunoglobulin [61]. Since there are currently no randomized trials on this, the data supporting the use of these immunomodulators for treating ILD is confined to case series, resulting in restricted confidence.
2. Nonpharmacotherapies
Symptom management, pulmonary rehabilitation, and preventive strategies are all essential aspects of care for patients with ILD. Although there have been recent advances in the pharmacotherapy of ILD, many patients still have limited treatment options [7]. Nonpharmacologic approaches are important for improving quality of life and reducing the symptoms of ILD [61]. Stopping smoking is essential for patients with PPF, as smoking is a risk factor for PPF [32]. Patients should receive influenzal, pneumococcal, and other age-appropriate vaccines.
Supplemental oxygen therapy should be recommended for patients with ILD who have resting or exercise-induced hypoxemia [65]. Ambulatory oxygen therapy can improve quality of life, endurance, and exercise performance, and can also help reduce anxiety and depression, which are often associated with chronic lung disease [61,66]. It can also improve the effectiveness of pulmonary rehabilitation by alleviating dyspnea on exertion and hypoxemia during the rehabilitation process [67].
Pulmonary rehabilitation may lead to significant benefits in patients with PPF. Pulmonary rehabilitation is a comprehensive intervention that includes education, exercise training, and behavior change, designed to improve the physical and psychological condition [68]. A Cochrane review of patients with ILD showed that those who underwent pulmonary rehabilitation were able to walk further, improve their maximum exercise capacity, and report less shortness of breath and improved quality of life for 2 to 6 months, compared to those who did not [69]. However, there were no significant differences in long-term effects, including survival [69]. A recent retrospective international cohort study found that patients with fibrosing ILD who demonstrated improvement in physical performance during pulmonary rehabilitation had better survival compared with those without improvement [70]. Although pulmonary rehabilitation has implemented aerobic training combined with strength training as the main intervention in many studies [71], further investigation is required to identify optimal exercise training regimens, educational topics, and intervention tailored to the complex needs of people with PPF.
Lung transplantation can be a life-extending treatment option for patients with advanced PPF [72]. The International Society of Heart and Lung Transplantation (ISHLT) guidelines recommended lung transplantation for patients with advanced lung disease that is not responding to medical therapy, who are at high (>50%) risk of death from lung disease, and who have a high (>80%) likelihood of surviving at least 5 years after lung transplantation [73]. While IPF is the most common indication for lung transplantation, PPFs, such as NSIP, CTD-ILD, LCH, occupational ILD, iPPFE, fibrotic HP, and sarcoidosis, have also been considered for transplantation [72]. In patients who underwent transplantation for end-stage ILD, older age and fibrotic NSIP were associated with a less favorable posttransplant survival. CTD-ILD showed posttransplant survival similar to IPF [74,75]. However, because these studies were retrospective and limited by selection bias, ISHLT considers CTD to be a risk factor for poorer outcomes and recommends screening for extrapulmonary disease in collaboration with a multidisciplinary team [72,73]. A retrospective study also revealed that two out of 11 patients who underwent lung transplantation with CTD-ILD experienced possible CTD flares during the follow-up period [74]. This is why the consideration of risk of flare-ups of underlying systemic inflammatory disease is also important in the context of lung transplantation in patients with CTD-ILD.
Conclusion
PPF is PF-ILDs other than IPF, with a poor prognosis (similar to IPF). As ILD can present as PPF, progression to PPF should be monitored through a combination of pulmonary function tests, symptom assessment, and CT scans, with regular follow-up. Although there is currently no known cure for PPF, there are treatments that can help slow the progression of the disease and improve quality of life. Continued research is needed to better understand PPF and develop more effective treatments.
Notes
Authors’ Contributions
Conceptualization: Song JW. Methodology: all authors. Formal analysis: all authors. Software: all authors. Validation: all authors. Investigation: all authors. Writing - original draft preparation: Kang HK. Writing - review and editing: all authors. Approval of final manuscript: all authors.
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Funding
This study was supported by grants from the Basic Science Research Program (NRF-2022R1A2B5 B02001602) and the Bio & Medical Technology Development Program (NRF-2022M3A9E4082647) of the National Research Foundation of Korea (NRF) funded by the Ministry of Science & ICT, Republic of Korea, as well as by grants from the National Institute of Health research project (2021ER120701) and the Korea Environment Industry & Technology Institute through the Core Technology Development Project for Environmental Diseases Prevention and Management Program funded by the Korea Ministry of the Environment (RS2022-KE002197), Republic of Korea.