The Current Status of BCG Vaccination in Young Children in South Korea
Article information
Abstract
Background
Delivery of Bacille Calmette-Guréin (BCG) Tokyo vaccine, with the multipuncture device, has been much preferred over BCG Pasteur, with the intradermal method, possibly due to the easier manner of administration, a desire to avoid any trouble with scars, as well as side effects and higher profits to providers in South Korea.
Methods
To determine BCG scar status in 0~6 year old children vaccinated with two BCG vaccines (Pasteur BCG vaccine with intradermal method and BCG Tokyo vaccine with percutaneous method), the data from the national BCG scar survey in 2006 was analyzed.
Results
Based on the national survey, the high proportion that were vaccinated with BCG Tokyo vaccines with the multipuncture method (64.5%) was noted in 0~6 year old Korean children. From inspection of scar formation, as an indicator of vaccination, the median number of the visible pin scars from the percutaneous method was 16 (interquartile range, 12~18) in the Korean children, and pin scars decreased as the age of the children increased (p<0.001).
Conclusion
The findings in this survey clearly showed a growing preference of parents for the BCG Tokyo vaccines by the multipuncture method in South Korea.
Introduction
Although Bacille Calmette-Guréin (BCG) vaccine is the only currently available vaccine against tuberculosis, polices for BCG vaccination vary between countries. Many countries use the intradermal method of administration for BCG vaccination recommended by World Health Organization (WHO) and United Nations Children's Fund (UNICEF), while some countries such as Japan (and South Africa until 2002, South Korea, Brazil) use the percutaneous method of administration with a multipuncture technique. Brazil was the last country to use oral administration of BCG vaccine until this was discontinued in 1973. Other techniques such as scarification, jet injection and use of bifurcated needles have been tried, but have yielded highly variable and inadequate results mainly because of inconsistent dose delivery, adverse reactions and low tuberculin conversion rate1. In South Korea, the policy is to give BCG vaccination within the first month of birth along with revaccination given to some elementary children aged 6 to 7 years if they have no visible BCG scar without prior tuberculin testing (until the revaccination policy was stopped in 2008). All the BCG vaccine needed in the nation had been produced by the Korean Institute of Tuberculosis (KIT) until the year 2007. However as per Korea's liberalized import action since the 1990s, imported BCG vaccines, such as the BCG Tokyo vaccine given by multipuncture device, have been also introduced to private health care providers. Use of the BCG Tokyo vaccine delivered by multipuncture device has been constantly increasing because it is known to be less scarring and to have lower rates of side effects compared to the BCG Pasteur vaccine given by the intradermal method2,3. Also, the private sector prefers to use the imported vaccines not only due to the higher profits, but also due to the easier means of administration which does not require special training.
Percutaneous administration methods are simpler with a low rate of adverse reactions but are less consistent in terms of delivery dose4. Intradermal injection using a syringe and needle is generally accepted as the most accurate method, because the delivered dose can be precisely measured and controlled, resulting in more consistent mycobacteria-specific immunity5,6. The downside of using the intradermal method is a higher rate of local reactions such as ulcers and lymphadenitis than other methods when other vaccine specific factors are controlled5. The implications of intradermal versus percutaneous administration routes have long been debated but, the relative protective efficacy of the two is not yet known. However, there have been some studies that compared the effect of vaccine route on T cell immunity against tuberculosis. Kemp et al.6 demonstrated that Mycobacterium tuberculosis-specific interferon-γ (IFN-γ) production was increased after the intradermal route of BCG vaccination but not by the percutaneous route in healthy volunteers. Also, Hussey et al.7 in South Africa demonstrated that there was a trend for greater IFN-γ responsiveness following the intradermal route of vaccination compared with percutaneouly administered BCG vaccine. On the other hand, in contrast with those of Kemp et al.6, a recent study in South Africa found that percutaneous vaccination with Japanese BCG induced significantly greater helper T cell (Th)-1 cytokines in whole blood of neonates than did intradermal vaccination with Danish BCG8. These studies with different conclusions suggest that the vaccine route can affect M. tuberculosis-specific T cell immunity in terms of IFN-γ and other T cell-derived cytokines, but it might be important to determine its actual effect on the variable vaccine efficacy and clinical consequences of vaccination in particular populations.
Therefore, it is necessary to investigate the current status of BCG vaccination in young children who have been affected by the diversity of BCG vaccines in use in the public and private sector in South Korea. Using the data from this BCG scar survey, we can evaluate whether proper BCG vaccination delivered by the two different administration methods has been performed in the nation. Findings from this study will provide baseline data to health policy decision makers to assist in evaluating the performance of the existing BCG vaccination program and to provide a better understanding of the BCG vaccines recently used in South Korea.
Materials and Methods
1. Study population
During 2006, as part of the National Tuberculosis (TB) Control Program of the Korea Center for Disease Control and Prevention (KCDCP) and the KIT, a BCG scar survey was carried out with technical assistance from the regional public health care centers and the KIT. Those children aged 0~6 years old were recruited from day-care centers and kindergartens, randomly selected by one-stage cluster sampling from every 16 cities and provinces of South Korea. Selected day-care centers or kindergartens which refused to participate were excluded, and a total of 121 institutions took part in the survey in the end.
This study was ethically approved by the Institutional Review Board of the International Vaccine Institute, Seoul (protocol # 2006-006). The parents or guardians of all subjects gave a written consent form for their children to participate.
2. BCG scar survey
Prior to inspection of BCG scar formation, a letter containing a written consent form and questionnaire asking about history of BCG vaccination, type of BCG vaccines, and family history of TB was distributed to parents/guardians of all subjects.
The health care workers inspected BCG scars by examining the left and right upper arms or elsewhere, by measuring BCG scar diameter (BCG Pasteur) and counting the number of puncture scars (BCG Tokyo). The BCG scar was recorded as 'intradermal method', 'multipuncture method' or 'no scar.' The presence of a BCG scar(s) was accepted as evidence of previous BCG vaccination regardless of the presence of parental report of BCG vaccine.
3. Statistical analysis
Data was analyzed using Stata software version 11.0 (Stata Corp., College Station, TX, USA). Comparisons were made between groups to test for statistical evidence of differences. Chi squared tests were used to compare different groups according to which vaccine they received. In addition, the non-parametric analysis, Kruskal-Wallis test and Wilcoxon rank sum test, was used to compare the scar size and pin scar numbers in different age groups.
Results
1. Characteristics of participants by the type of BCGs
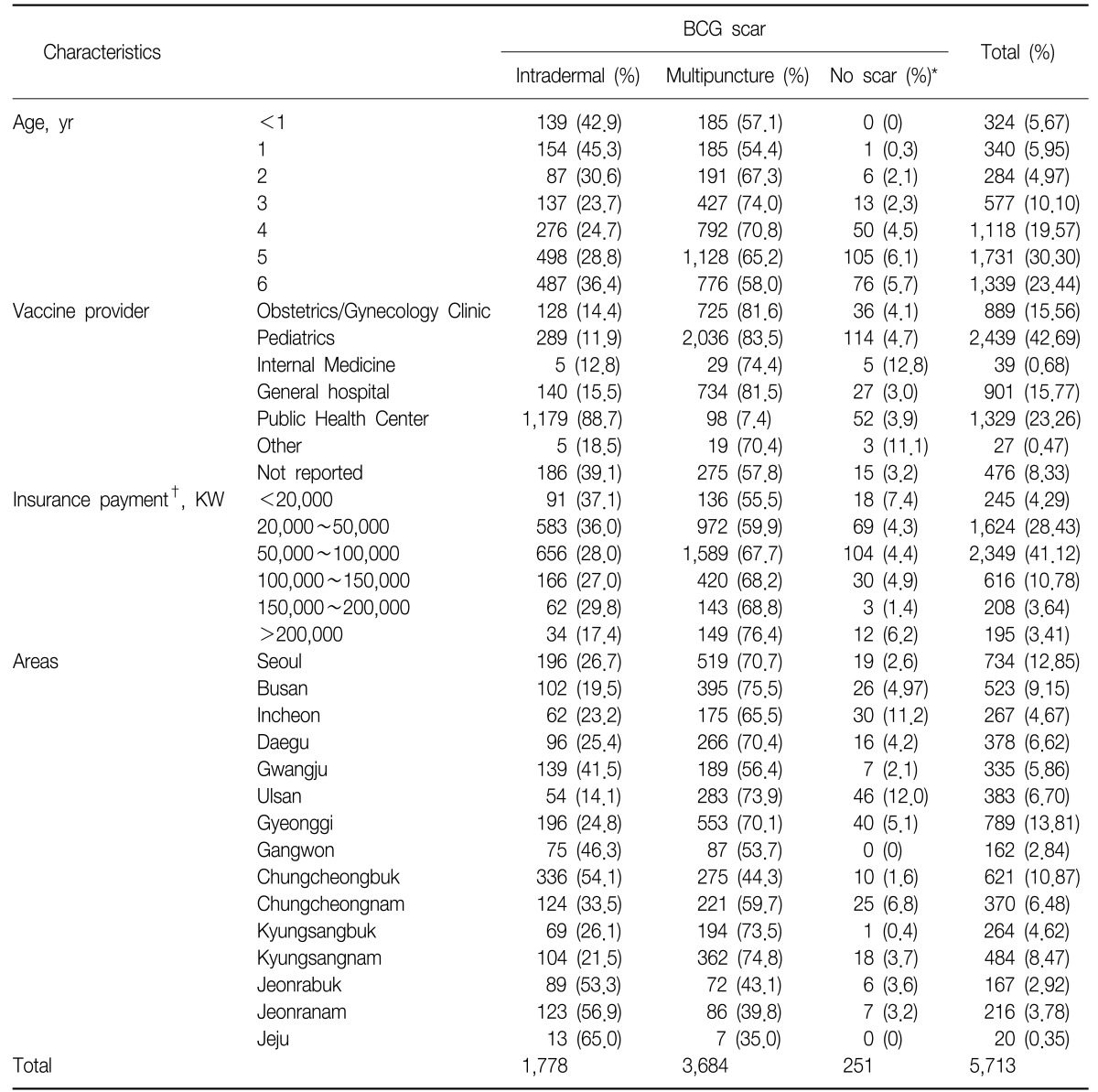
For the investigation of BCG scar formation, the data from a total of 5,713 children were used, collected from 121 day-care centers and kindergartens in 16 cities of South Korea. From the information collected for those children whose parents responded to the questionnaire, there were 2,995 male and 2,718 female children with a ratio 1.1 to 1. Among the 5,713, for 95.6% (n=5,462) the parent indicated that the child had been vaccinated with BCG.
The distribution of BCG scar status in the Table 1 was categorized primarily by the data collected from scar inspection in the field. When an examiner recorded the child as 'no BCG scar' during the inspection but the parents had ticked vaccination with one of BCGs in the questionnaire, the answer from the parents was used. However, when an examiner recorded the child as 'no BCG scar' during the inspection and the parents marked 'not sure' for BCG status in the survey, then this was categorized as 'no scar' in the analysis.
For those children who had been inspected for BCG scar, 1,778 (31.1%) had been vaccinated with BCG Pasteur by the intradermal method and 3,684 (64.5%) with BCG Tokyo by the multipuncture method. From this analysis, 251 children (4.4%) had 'no BCG scar' with an increasing trend by age.
Among girls, 31.6% had been vaccinated with BCG Pasteur by the intradermal method (n=817) and 68.4% with the BCG Tokyo by the multipuncture method (n=1,769). Among boys, 15.9% had been vaccinated with BCG Pasteur by the intradermal method (n=361) and 84.1% with the BCG Tokyo by the multipuncture method (n=1,915).
The distribution of the type of BCGs varied by age, showing the proportions of BCG given with the intradermal method were from 45.3% to 23.7% (p<0.0001). It is noted that children less than 2 years old had relatively higher proportions of BCG Pasteur with the intradermal method than had been expected. Looking separately at the data collected from day-care centers and kindergartens, the proportion of BCG Pasteur vaccination with the intradermal method increased by age, indicating that the proportion of children vaccinated with BCG Tokyo with the multipuncture method was gradually increasing in recent years.
Analysis of the providers of BCG vaccination, in the case of children given BCG Pasteur/intradermal, 88.7% of responders said that their children had been vaccinated in public health care centers. On the other hand, the data showed that private clinics preferred to provide BCG Tokyo/multipuncture.
Interestingly, 7.4% of parents/guardians of BCG Tokyo vaccinees responded in the survey that their children had received the vaccine in public health care centers, where the BCG Tokyo vaccine is not provided. This may be due to recall bias or inaccuracy in recording, given that in the private sector vaccination is also provided from a public health center. Compared to the other private clinics, pediatrics clinics had a significantly lower proportion of vaccination with BCG Pasteur/intradermal as 11.9%.
To observe any association with the economic status of parents/guardians, information on monthly insurance payments in proportion to their incomes was collected in the survey. The data showed that those parents/guardians who paid the higher insurance payments (which indicates higher incomes) had the higher proportion of vaccination with BCG Tokyo/multipuncture (p<0.0001).
In terms of the regional preference of the type of BCGs, 35.0% of children living in Jeju had been vaccinated with BCG Tokyo/multipuncture while 75.5% of children living in Busan had been vaccinated with the same vaccination, showing that the big cities with higher income had a relatively high proportion of BCG Tokyo/multipuncture than other smaller cities. Since BCG Tokyo/multipuncture is more expensive than BCG Pasteur/intradermal which is free of charge, it seems children in bigger cities had more access to the more expensive BCG Tokyo vaccine.
2. BCG scar formation
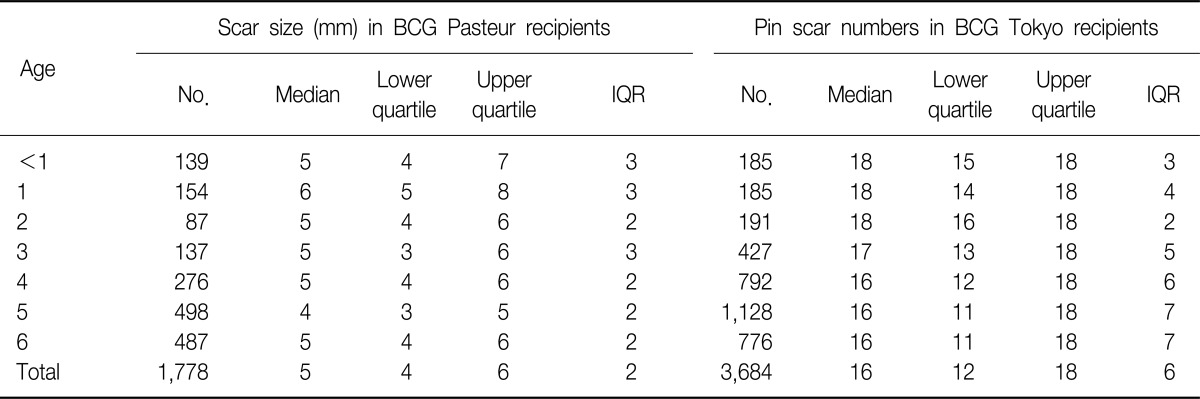
Among those children who had been vaccinated with BCG Pasteur by the intradermal method at the age of 0 to 6 years (n=1,778), the overall median of the BCG scar size was 5.0 mm (interquartile range [IQR], 1~15). According to the non-parametric analysis in Table 2, the data shows that the medians of scar size were significantly different by age (Kruskal-Wallis test, p<0.001), especially the older age group (4~6 years) had significantly higher scar sizes than the younger age group (0~3 years) (Wilcoxon rank sum test, p<0.001). There were 75 children (4.2%) who had scar sizes of more than 10 mm, which needs further investigation as to whether this was due to keloid formation or recording errors. From observation of the distribution in scar size, it is noted that there are distinct peaks at 5 and 10 mm (Figure 1). It might be possible that examiners from public health care centers had 'digital preferences' when measuring the size of BCG scar at the inspection.

Distribution of scar size (mm) among BCG Pasteur recipients by age. The figure excludes data from children with no scar. The different lines indicate each age group from 0 year to 6 years old. BCG: Bacille Calmette-Guréin.
Among those children who had been vaccinated with BCG Tokyo/multipuncture, the number of BCG pin scars was recorded at ages of 0 to 6 years from the scar survey (n=3,684). For all the children, the median of pin scars was 16 (IQR, 12~16), the maximum number of pin scars was 36, and the minimum number was 1. According to the non-parametric analysis in Table 2, the data shows that the medians of pin scar number were significantly different by age (Kruskal-Wallis test, p<0.001), especially the younger age group (0~3 years) had significantly higher pin scars than the older age group (4~6 years) (Wilcoxon rank sum test, p<0.001).
Discussion
From this scar survey, 4.4% of responders had no BCG scar. Based on the national tuberculosis survey in 1995, the rate of BCG vaccination under 10 years old was 87.7%9. Comparing these data, it seems the rate of BCG vaccination has improved since 1995. However, the reporting rate of BCG vaccination has been slightly decreasing, taking into consideration the figures of 80.3% in 2002, 77.8% in 2003, and 77.7% in 2004. A lot of individuals on whom data were collected may not have been properly reported to the surveillance program, which has no system of collecting data from all the private clinics. This suggests that the current reporting system should be examined and improved so as to include information on all vaccinated children.
From the data for the age of 0 to 6 years, 64.5% of responders had been vaccinated with BCG Tokyo/multipuncture. It appears that only 11.8% of responders in pediatric clinics and 14.4% of responders in obstetrics/gynecology clinics, had vaccination with BCG Pasteur/intradermal, showing that most of the private clinics prefer to use the BCG Tokyo vaccine. It seems the use of BCG Tokyo by the multipuncture method in private sector has been gradually increasing compared to that of BCG Pasteur given by the intradermal method. In addition to an easier manner of administration, less scarring and higher profits to the providers, the private sector may also favor using the BCG Tokyo strain, considering the fact that BCG Tokyo strain is known to be one of the 'weak' BCG strains with lower frequencies of side effects such as BCG lymphadenitis than 'strong' strains such as BCG Pasteur and BCG Danish10,11.
As the multipuncture method uses a device with 9 needles twice, a maximum of eighteen BCG scars should remain in the skin when properly injected, but previous studies have found that typically only 9 to 11 BCG scars remained a few years after vaccination12,13. In this survey, the data showed that the median of pin number scars was 16 (IQR, 12~18) among 3,684 BCG Tokyo recipients at the age of 0~6 years. In children less than 12 months old, the median of pin number was 18 (IQR, 15~18), while the median of pin number in 6 years old was 16 (IQR, 11~18), showing a decreasing trend of pin scars over time. This may provide further evidence of the disappearance of pin scars over time, although we cannot discount the possibility that as the use of BCG delivered with the multipuncture device increased over time, the injection technique improved, which may have resulted in increased numbers of pin scars in younger children.
The findings in the survey clearly showed a growing preference of parents for the BCG Tokyo vaccine by the multipuncture method in South Korea. This suggests that special training and education to ensure accurate injection in the private sector will have to be planned by the government.
Acknowledgements
This project was financially supported by the International Tuberculosis Research Center, Masan, Korea.