Quantitative PCR for Etiologic Diagnosis of Methicillin-Resistant Staphylococcus aureus Pneumonia in Intensive Care Unit
Article information
Abstract
Background
Ventilator-associated pneumonia (VAP) requires prompt and appropriate treatment. Since methicillin-resistant Staphylococcus aureus (MRSA) is a frequent pathogen in VAP, rapid identification of it, is pivotal. Our aim was to evaluate the utility of quantitative polymerase chain reaction (qPCR) as a useful method for etiologic diagnoses of MRSA pneumonia.
Methods
We performed qPCR for mecA, S. aureus-specific femA-SA, and S. epidermidis-specific femA-SE genes from bronchoalveolar lavage or bronchial washing samples obtained from clinically-suspected VAP. Molecular identification of MRSA was based on the presence of the mecA and femA-SA gene, with the absence of the femA-SE gene. To compensate for the experimental and clinical conditions, we spiked an internal control in the course of DNA extraction. We estimated number of colony-forming units per mL (CFU/mL) of MRSA samples through a standard curve of a serially-diluted reference MRSA strain. We compared the threshold cycle (Ct) value with the microbiologic results of MRSA.
Results
We obtained the mecA gene standard curve, which showed the detection limit of the mecA gene to be 100 fg, which corresponds to a copy number of 30. We chose cut-off Ct values of 27.94 (equivalent to 1×104 CFU/mL) and 21.78 (equivalent to 1×105 CFU/mL). The sensitivity and specificity of our assay were 88.9% and 88.9% respectively, when compared with quantitative cultures.
Conclusion
Our results were valuable for diagnosing and identifying pathogens involved in VAP. We believe our modified qPCR is an appropriate tool for the rapid diagnosis of clinical pathogens regarding patients in the intensive care unit.
Introduction
Hospital-acquired pneumonia (HAP) is the second most common nosocomial infection1. HAP is 6~20 times more common in mechanically-ventilated patient, and ventilator associated pneumonia (VAP) occurs in 9~27% of all intubated patients2,3. The mortality rate of VAP has been reported to range between 33% and 50%4. Because of increasing mortality, most patients with pneumonia are treated with broad-spectrum antibiotics, and this treatment increases hospital cost as well as the prevalence of resistant strains5. To minimize the risk of mortality, clinicians are recommended to avoid delaying administration of appropriate antibiotic treatments6.
The National Nosocomial Infection Surveillance from 2003 found that Staphylococcus aureus was the most common pathogen associated with VAP (27.8%) in US1. Methicillin resistance is caused by the penicillin-binding protein 2a (PBP2a), which is encoded by the methicillin-resistant Staphylococcus aureus (MRSA) mecA gene. Because genes for methicillin resistance in Staphylococcus aureus are well-known, mecA is used to differentiate between methicillin-sensitive Staphylococcus aureus (MSSA) and MRSA7.
Because the results of a microbiologic culture were not immediately available, empirical antibiotic therapy could lead to inadequate antibiotic treatment. Molecular-based diagnostic methods, such as quantitative polymerase chain reaction (qPCR), have been evaluated for their ability to identify MRSA by detecting the mecA gene8-10. This has the potential to deliver rapid, specific and sensitive detection. Previously, a study with the qPCR of blood indentified sepsis-relevant microorganisms11. Because qPCR for MRSA was limited to surveillance with nasal swabs, it was impossible to determine whether the specimens were true pathogens or colonization12. Multiplex PCR to detect Gram negative bacilli could not discriminate true pathogens, previously13. qPCR have not been completely evaluated for their ability to provide etiologic diagnostic criteria for clinical specimens14,15.
The object of this study was to develop and validate a qPCR assay from MRSA detection on bronchoalveolar lavage (BAL) or bronchial washing samples.
Materials and Methods
1. Specimen preparation and extract clinical data
This study was performed at a 52-bed intensive care unit (ICU) in Konyang University Hospital (Daejeon, Korea). Samples consisting of a BAL and bronchial washing samples were collected directly from patients admitted to the ICU. The study included 72 clinical samples that consisted of 72 VAP episodes in 64 patients, during a period of 11 months (March 2010 to February 2011). The study was approved by the Institutional Review Board of Konyang University Hospital, and we received informed consent from all patients.
2. BAL and bronchial washing samples
We obtained clinical samples from patients who had "clinically-suspected pneumonia" that was defined as follows: patients received mechanical ventilation for at least 48 hours in the ICU and had new or progressive infiltration on chest X-rays. In addition, patients had at least two of following symptoms indicating infection: 1) temperature<36℃ or ≥38.3℃, 2) white blood cell <5,000 cells/mm3 or >10,000 cells/mm3, and 3) purulent sputum4.
BAL samples were taken from pulmonary subsegments and bronchial washing samples were taken, using fiberoptic bronchoscopy from a segment or lobe in which new or progressive infiltration had developed. If lesions were not identified, samples were taken from basal segments of lower lobe. We performed the BAL procedure and bronchoscopy methods standard in the ICU16.
3. Identification and antimicrobial susceptibility: breakpoint by Clinical and Laboratory Standards Institute guidelines
Quantitative cultures of BAL or bronchial washing specimens were performed in a microbiology laboratory. The specimens were inoculated in blood and MacConkey agar plates with a 0.001 mL-loop. After incubation at 37℃ in a 5% CO2 incubator, for 24 hours, a colony count was performed and expressed as the number of colony-forming units per mL (CFU/mL). In general, the number of CFU/mL is equal to the number of colonies on the agar plate multiplied by the inoculation factor. In our quantitative culture, the pathogen was evaluated by the diagnostic criteria for BAL specimens (≥1×104 CFU/mL) and bronchial washing specimens (≥1×105 CFU/mL). Organisms isolated from specimens were confirmed using Combo Panels of the MicroScan WalkAway 96 system (Dade Behring, West Sacramento, CA, USA) for antimicrobial susceptibility and species identification.
4. DNA extraction
To extract DNA from BAL or bronchial washing samples, we used a NucleoSpin® Tissue extraction kit (MACHEREY-NAGEL, Neumann-Neander-Str., Germany), closely following the manufacture's protocols. The clinical sample volume was 1 mL, and an equal volume of N-acetyl/NaOH was added to the sample. After incubating the mixture for 25 minutes at room temperature, while shaking it, the mixture volume was adjusted to 25 mL with sterile water and the mixture was centrifuged at 4,000 ×g for 30 minutes. The supernatant was discarded, two hundred µL of buffer T1 was used to re-suspend the pellet, and 25µL of proteinase K was added. For inhibitor control, 100 pg of spiked an internal control (SPUD) (Table 1) was also added. The samples were incubated at 56℃ and were shaken until complete lysis was obtained. Two hundred µL of buffer B3 was added for lysis, and the sample was incubated at 70℃ for 10 minutes. Then, 210µL of ethanol (96~100%) was added, and the sample was placed on a NucleoSpin® tissue column, in a collection tube. After centrifugation for 1 minute at 11,000 ×g, the flow-through was discarded, and 500µL of buffer BW were added for first wash. The sample was then centrifuged for 1 minute at 11,000 ×g. Next, 600µL of buffer B5 were added to the column for the second wash and centrifuged for 1 minute at 11,000 ×g. The flow-through was the discarded, and the column was centrifuged for 1 minute at 11,000 ×g in order to remove residual ethanol. Then, 50µL of pre-warmed elution buffer BE (70℃) was added, and the sample was incubated at 25℃ for 1 minute. After centrifugation for 1 minute at 11,000 ×g, the extracted DNA preparations were adjusted to a final volume of 50µL.
5. Reference strains
Three reference strains of Staphylococcus spp., including methicillin-resistant and, methicillin-susceptible strains, were included in the study. The strains were MRSA (ATCC 33591), MSSA (ATCC 25923), and methicillin-sensitive Staphylococcus epidermidis (MSSE) (ATCC 12228). All strains were provided by Kyungbook National University Hospital and the Korean Federation of Culture Collections, Korean Culture Center of Microorganisms.
6. Quantitative PCR
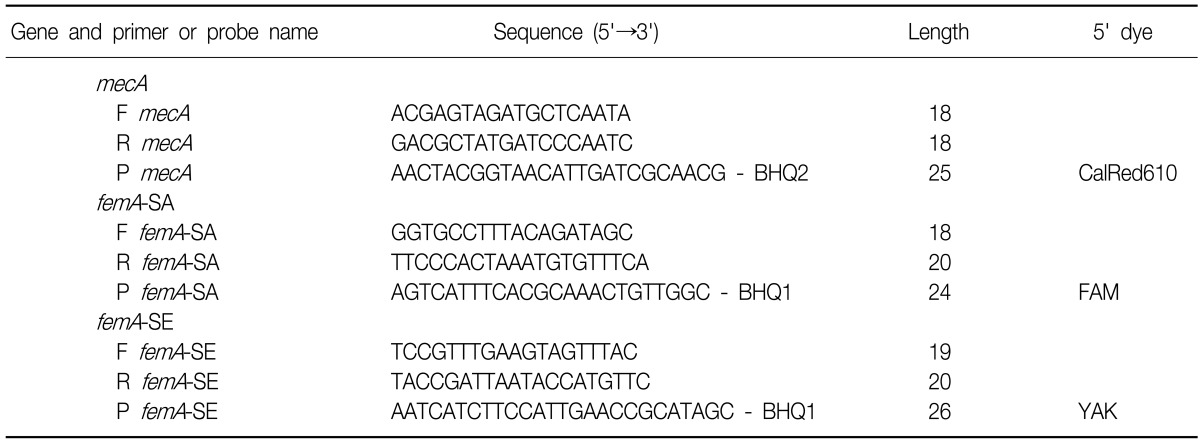
Multiplex PCR was performed in a 20µL volume that contained 10µL of iQ™ Supermix (Bio-Rad, Hercules, CA, USA), 125 nM of each primer, 125 nM probes, and 4µL of template. The amplification procedure for the Bio-Rad iQ5 took place at 95℃ for 5 minutes, followed by 50 cycles at 95℃ for 10 seconds and 57℃ for 30 seconds with, fluorescence acquisition after every cycle. The sequence of the TaqMan primers and probes for MRSA qPCR was based on work from Francois et al.17 and modified by the Beacon Designer (Premier Biosoft, San Diego, CA, USA) software, as described in Table 2.
Using this method, detection of only the femA-SA indicated the presence of MSSA, and detection of both the femA-SA and mecA genes indicated the presence of MRSA. If only the femA-SE gene was detected, the presence of MSSE was inferred. Furthermore, the detection of femA-SE and mecA genes, or the detection of the mecA gene alone, indicated the presence of methicillin-resistant coagulase negative Staphylococci (MRCNS).
7. Statistics
Data are shown as the mean±SD. Differences between the two groups were assessed using the student's t-test.
Results
1. Result of microbiologic cultures of clinical samples
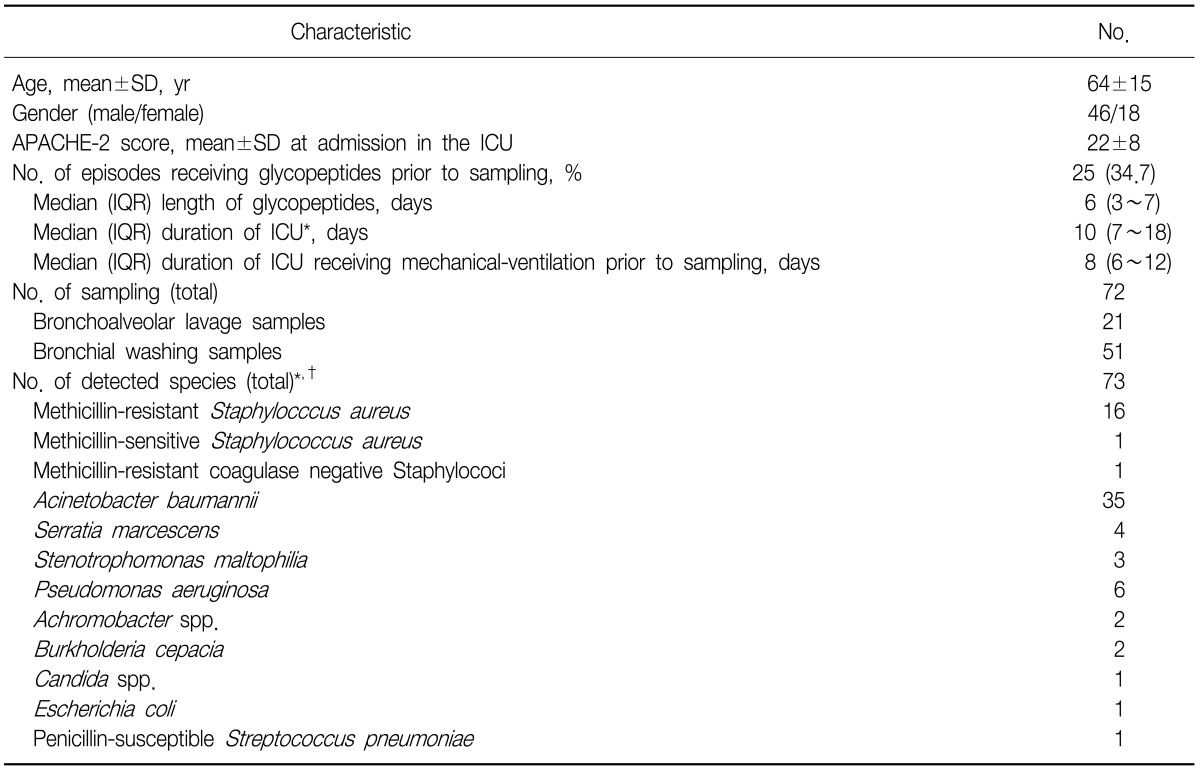
The assay was applied to 72 clinical samples of 64 patients from medical (56.3%), surgical non-traumatic (15.6%) and surgical traumatic (28.1%) categories. Reasons for mechanical ventilation were neurologic diseases (31.3%), pulmonary diseases (29.7%), postoperative respiratory failure (12.5%) and the others (26.5%). The clinical characteristics and the identified species from patients are described and summarized in Table 3.
2. Measure reproducibility and obtained standard curve about mecA, femA-SA, and femA-SE genes
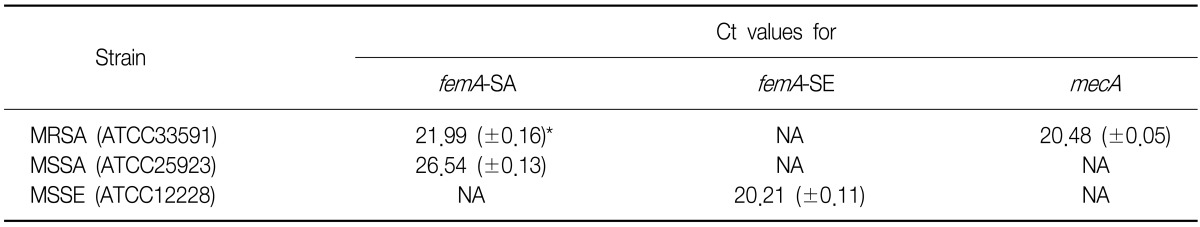
We measured the reproducibility of detecting the femA-SA, femA-SE and mecA genes in the reference strains (Table 4).
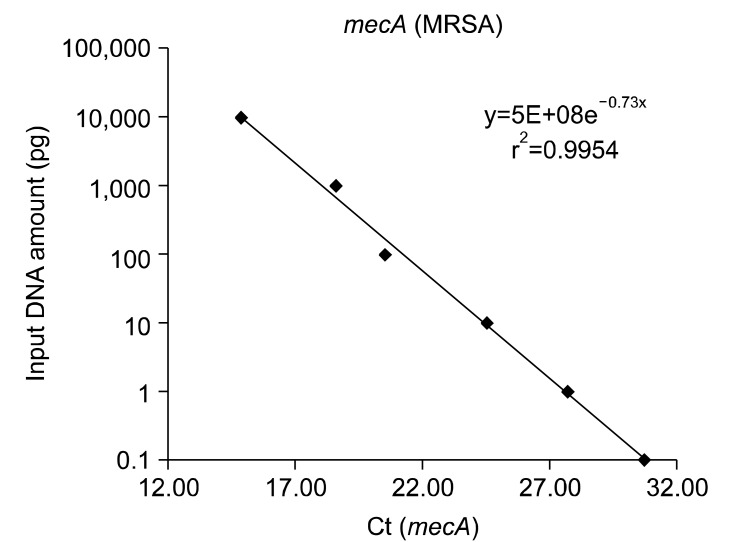
We obtained the mecA gene standard curves by serial dilution of genomic DNA under the assumption that 1 copy number of the mecA gene corresponds to 1 CFU18. The results indicate that the detection limit of the mecA gene is 100 fg:30 copy number (Figure 1).
3. Comparison between qPCR results and quantitative culture results
We detected MRSA, MSSA, and MRCNS using the qPCR. As a standard curve (Figure 1), we determined the cut-off Ct value (28.94) that corresponded to diagnostic criteria (1×103 CFU/mL) and compared the results of qPCR with results from the quantitative culture. We concluded that the sensitivity and specificity were 94.11% and 74.54%, respectively (data not shown). Additionally, we chose a quantitative microbiological method based on the inoculation in plates with a 0.001 mL-loop. This method did not allow for a culture of less than 103 CFU/mL.
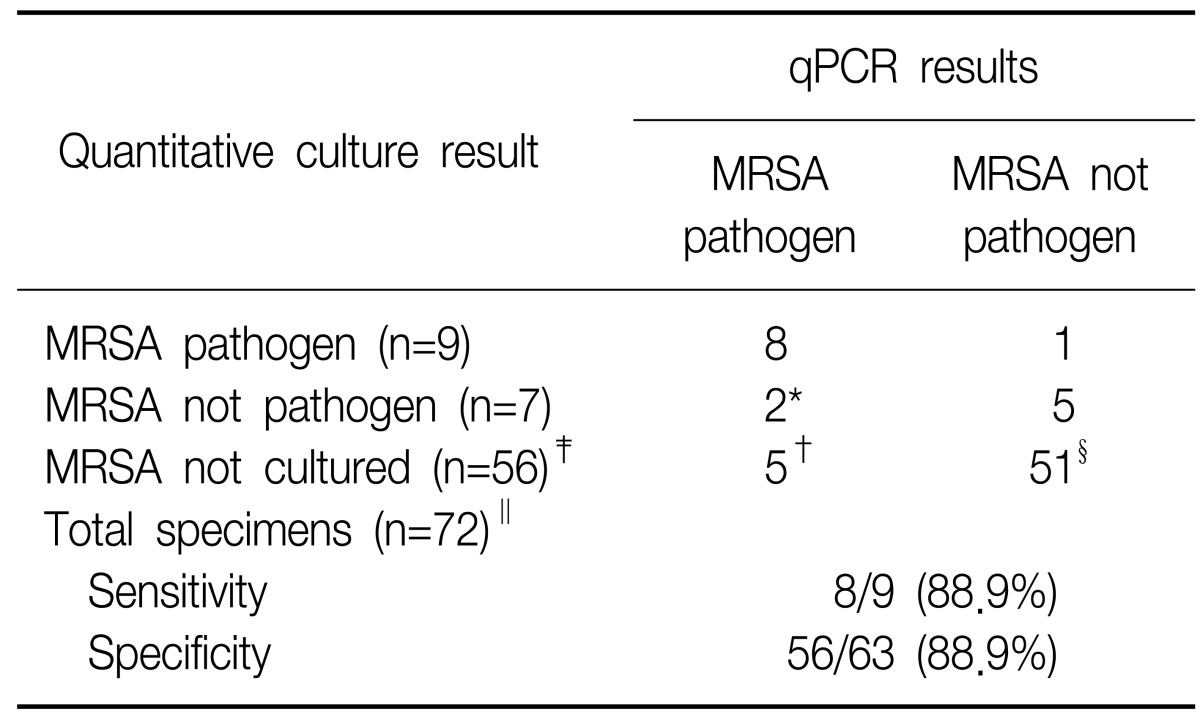
After discriminating between MRSA cultures and other specimens for determining the presence of pathogens, we divided the samples into four groups, based on the CFU results from quantitative cultures: group 1 contained samples with culture negatives, group 2 contained samples with cultures of ≥103 to <104 CFU/mL, group 3 contained samples with cultures of ≥104 to <105 CFU/mL, and group 4 contained samples with a culture of ≥105 CFU/mL. The Ct values were 23.97±3.97 for group 3 and 18.72±3.06 in group 4 (Figure 2). The difference between group 3 and group 4 was statistically significant (p<0.05). In the standard curve of the mecA gene, the corresponding Ct to CFU values were 2.08×103 to 6.05×105 CFU/mL for group 3 and 1.87×105 to 1.65×107 CFU/mL for group 4. To minimize the differences between reference MRSA strains and extracted specimens, and to reduce false-positives, we determined that a cut-off value of 27.94 (mean+SD in group 4) corresponded to 1×104 CFU/mL, and a cut-off value of 21.78 (mean+SD in group 2) corresponded to 1×105 CFU/mL. To define whether the specimens were pathogens or colonization, we used a cut-off value such that sample Ct values of ≤27.94 from BAL specimens and ≤21.78 from bronchial washing specimens were diagnosed as pathogens. These cut-off values resulted in a sensitivity of 88.9% and a specificity of 88.9% (Table 5). With the exception of the 25 VAP episodes that received antibiotics for MRSA, we analyzed a total of 47 VAP episodes. qPCR assay had a sensitivity of 85.7% and specificity of 90.0% for the pre-antibiotic samples. There were 4 false positives and 1 false negative in the qPCR assay.

Box-plot of Ct values from methicillin-resistant Staphylocccus aureus (MRSA) cultured specimens. Of the total samples, we created 4 groups based on the colony-forming units (CFU) results from the quantitative culture. Group 1 (n=56) contained samples with negative cultures, group 2 (n=0) contained samples with cultures of ≥103 to <104 CFU/mL, group 3 (n=8) contained samples with cultures of ≥104 to <105 CFU/mL, and group 4 (n=8) had samples with a culture of ≥105 CFU/mL. Boxes represent the 25th to 75th percentile interquartile range, with the line in the middle representing the median. The upper horizontal line represents the cut-off value of 1×104 CFU/mL, and the lower horizontal line represents the cut-off value of 1×105 CFU/mL.
Discussion
According to data from the Korean Nosocomial Infection Surveillance System, MRSA is the pathogen most frequently isolated in VAP19. Mortality caused by MRSA pneumonia ranges between 14% and 47% but is significantly lower among patients receiving early appropriate antibiotic treatment than in those requiring a change in treatment20. Recent studies have correlated qPCR with quantitative culture counts and have provided therapeutic information for applying diagnostic criteria (1×104 CFU/mL) to BAL specimens14. To indicate appropriate therapeutic information, we correlated firstly qPCR results with clinical pathogens. Therefore, we not only reduced differences between qPCR and quantitative cultures but also suggested a clinical setting for bronchial washing specimens.
We estimated the number of MRSA CFUs isolated from BALs or bronchial washing specimens and compared the results of qPCR with the results of quantitative cultures. In our study, 1 of 72 clinical samples (1.4%) was classified as a false-negative (Table 5) and this patient may have received inappropriate treatment leading to fatality. The patient's clinical pulmonary infection score (CPIS) was greater than 7 and MRSA grew more than 105 CFU/mL. Combining the CPIS score with qPCR results would likely have yielded a more accurate diagnosis for this patient.
Seven patients' (qPCR, MRSA pathogen and quantitative culture, MRSA not pathogen [2 patients]; qPCR, MRSA pathogen and quantitative culture, MRSA not cultured [5 patients]) (Table 5) results classified as false-positives. Of the 7 false-positives, five patients' specimens (qPCR, MRSA pathogen and quantitative culture, MRSA not cultured) (Table 5) had no MRSA in a quantitative culture. According to their clinical records, all patients had "clinically-suspected VAP" and CPIS was exceeded 6. Two of the five patients received glycopeptides within the 48 hours prior to obtaining samples. One patient who maintained vancomycin was survived, but the remaining patients with acute respiratory distress syndrome did not respond to teicoplanin. The qPCR method might not distinguish nonviable organisms from viable ones, because the DNA of some nonviable organism could still be extracted21,22. We should consider recent antibiotic therapy before extracting the specimens for qPCR, because patients who showed a favorable clinical response to therapy had a rapid fall in colony counts within 24 to 72 hours23. One patient with high risk for MRSA pneumonia, whose medication was changed to teicoplanin, showed improvement. Two patients who had not received glycopeptides progressed. Five patients might have had MRSA, so our qPCR method might be more sensitive than a microbiologic culture.
Two patients (qPCR, MRSA pathogen and quantitative culture, MRSA not pathogen) (Table 5) who had clinically-suspected VAP had MRSA that was less than the cut-off value for microbiologically-diagnosed VAP. One patient receiving glycopeptides improved, which suggests that the result of the qPCR were accurate. The other patients' results were false positives. One of seven patients, according to positive results of qPCR (1/72 VAP episodes, 1.4%), might have taken unnecessary glycopeptides during our study. In contrast, six of the seven patients with negative or low levels of CFU/mL of microbiologic cultures might not have received glycopeptides for MRSA pneumonia, which might have contributed to an unfavorable outcome.
In our study, 51 specimens were bronchial washing samples that were easier to obtain, and the less invasive to critically ill patients, than BAL or protected specimen brush (PSB). The quickest, easiest and least expensive sample to obtain was the endotracheal aspirate. We used, rather than blind sampling, bronchoscopic sampling; blind aspiration sampling could lead to errors and diagnosis could be missed in some patients16. Although washing samples were obtained from the distal portion of lobar bronchus and involved VAP, we used a cut-off value established by the endotracheal aspirate diagnostic criteria (≥1×105 CFU/mL) instead of BAL (≥1×104 CFU/mL), owing to misleading colonization to pathogen by using a low-cut off. In previous studies, when a bacterial count ≥1×105 CFU/mL showed positive culture results of endotracheal aspirate, the sensitivity and specificity were 80% (range, 60~97%) and 62% (range, 41~74%) respectively. There was no difference between the several modalities of sampling in obtaining clinically significant pathogens24,25. In addition, the Ct values of qPCR were well-correlated with the quantitative culture of specimens. When using the diagnostic criteria of ≤27.94 (equivalent to 1×104 CFU/mL) in BAL specimens and ≤21.78 (equivalent to 1×105 CFU/mL) in bronchial washing specimens, the sensitivity was 88.9% and the specificity 88.9%.
The major finding of our study was that the qPCR method can be used to confirm whether colonized MRSA is a pathogen involved in VAP. A limitation is that it is difficult to differentiate patients infected only with MRSA from those who are co-infected (MSSA and methicillin-resistant Staphylococcus epidermidis). However, the co-infected patients are clinically uncommon cases.
We used a modified SPUD assay to evaluate experimental and clinical conditions26. We spiked the SPUD in the specimen and used a high-throughput column method of DNA extraction. This methodology allows the qPCR method to be utilized routinely. The recovery rate of this method was not constant, as in sample conditions: agglutination of sputum. For this reason, we needed to consider the recovery rate for absolute quantification. With this modification, we were able to consider not only inhibitors but also technical errors. Thus, the process more accurately reflected clinical conditions.
Diagnosis of VAP was very difficult in the ICU patient. Instead of developing a clinical suspected scoring system, diagnostic criteria and microbiology diagnosis were used4. Our study shows that the qPCR could be good for diagnosing pneumonia and might be an appropriate substitute for microbiologic cultures. In a study of PSB in suspected VAP, quantitative cultures led to antimicrobial changes in 38% of the patients with inadequate initial therapeutic plans27. In another study, patients with clinically-suspected VAP had a high mortality rate regardless of whether BAL cultures confirmed a clinical diagnosis of VAP28. When adequate antibiotic therapies were initiated very early, the mortality rate was reduced when compared to adequate therapies that were delayed until bronchoscopies were performed or BAL results were known29. Rapid turnaround times are therefore highly important in diagnosing and treating VAP. In our study, we not only demonstrated a rapid qPCR method to diagnose whether there were pathogens, but also reduced differences between qPCR and quantitative culture results. In addition, we suggested clinical settings for bronchial washing specimens, which had not yet been established. In conclusion, our results were valuable for diagnosing and identifying pathogens for VAP. We suggest that our modified qPCR is an appropriate and rapid tool for diagnosing clinical pathogens in ICU patients.
Acknowledgements
This study was supported by Konyang University, Myunggok Research Fund of 2010 (2010-16).