Factors that Predict Clinical Benefit of EGFR TKI Therapy in Patients with EGFR Wild-Type Lung Adenocarcinoma
Article information
Abstract
Background
Epidermal growth factor receptor (EGFR) mutations in non-small cell lung cancers have emerged as key predictive biomarkers in EGFR tyrosine kinase inhibitor (TKI) treatment. However, a few patients with wild-type EGFR also respond to EGFR TKIs. This study investigated the factors predicting successful EGFR TKI treatment in lung adenocarcinoma patients with wild-type EGFR.
Methods
We examined 66 patients diagnosed with lung adenocarcinoma carrying wide-type EGFR who were treated with EGFR TKIs. The EGFR gene copy number was assessed by silver in situ hybridization (SISH). We evaluated the clinical factors and EGFR gene copy numbers that are associated with a favorable clinical response to EGFR TKIs.
Results
The objective response rate was 12.1%, while the disease control rate was 40.9%. EGFR SISH analysis was feasible in 23 cases. Twelve patients tested EGFR SISH-positive, and 11 were EGFR SISH-negative, with no significant difference in tumor response and survival between EGFR SISH-positive and -negative patients. The overall median progression-free survival (PFS) and overall survival (OS) of 66 patients were 2.1 months and 9.7 months, respectively. Female sex and Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0–1 were independent predictors of PFS. ECOG PS 0–1 and a low tumor burden of extrathoracic metastasis were independent predictors of good OS.
Conclusion
Factors such as good PS, female sex, and low tumor burden may predict favorable outcomes following EGFR TKI therapy in patients with EGFR wild-type lung adenocarcinoma. However, EGFR gene copy number was not predictive of survival.
Introduction
Lung cancer is a leading cause of cancer-related death worldwide1. Approximately 85% of lung cancer cases are nonsmall cell lung cancer (NSCLC). The majority of patients are diagnosed with advanced disease; outcomes remain poor, with a median survival time of 8–10 months and a 2-year survival rate of 10%–20%23.
Recent advances in understanding the molecular biology of lung cancer have improved treatment of NSCLC through the development of targeted agents against molecular subgroups with specific genomic aberrations. Epidermal growth factor receptor (EGFR) has been one of the most important targets in the era of individualized therapy for NSCLC, leading to improvements in survival outcomes with a median survival of 24–30 months and an improved quality of life for patients with tumors harboring EGFR mutations, which are predominantly found in pulmonary adenocarcinomas456. EGFR mutations are found in approximately 10% of Caucasian patients with NSCLC and in 40%–50% of East Asian patients7. As for patients whose tumors have wild-type EGFR, only a subgroup achieves a favorable clinical response following EGFR tyrosine kinase inhibitor (TKI) treatment, suggesting that other factors may be involved. Thus, several biomarkers have been investigated in EGFR wild-type NSCLC. EGFR expression, EGFR amplification, EGFR ligand expression, and the involvement of other biomarkers that activate the EGFR signaling pathway are all associated with TKI sensitivity8. However, to date, there is no biomarker that clearly and reproducibly identifies patients with EGFR wild-type NSCLC who are likely to benefit from EGFR TKIs.
Recent studies have shown that a high EGFR gene copy number is associated with a better response to TKI therapy and improved survival9101112. An increase in EGFR copy number may dysregulate the activation of the EGFR tyrosine kinase receptor protein and trigger downstream oncogenic pathways13. The gold standard for detecting EGFR gene copy number is fluorescence in situ hybridization (FISH)14. However, the disadvantages of FISH are that it is labor-intensive and time-consuming, requires expensive equipment, and experiences a rapid loss of signal upon exposure to light. Silver-enhanced in situ hybridization (SISH) enables pathologists to evaluate slides using bright field microscopy with fully automated analysis in a cost-effective way15. Several studies reported a high concordance rate between SISH and FISH in terms of detection of EGFR amplification in NSCLC16 and glioblastoma17.
Sensitivity to EGFR TKI is associated with female sex, adenocarcinoma histology, no history of smoking, and an Asian background. However, such clinical factors are in fact predictors of EGFR mutations4. Therefore, there is insufficient information with which to identify patients with EGFR wild-type lung adenocarcinoma who would be eligible for TKI treatment.
This study aimed to determine whether an increased EGFR gene copy number, as evaluated by SISH, could be used to select patients with EGFR wild-type lung adenocarcinoma who are likely to benefit from EGFR TKI treatment. Furthermore, we explored clinical factors that influence the efficacy of EGFR TKIs in order to evaluate their potential applications to patient populations whose tumors are unlikely to carry EGFR mutations.
Materials and Methods
1. Patients
This retrospective study included patients with histologically confirmed stage IIIb, stage IV, or recurrent NSCLC who underwent EGFR mutation testing between February 2009 and December 2013 at a Korean Cancer Center Hospital. Eighty-four patients with EGFR wild-type lung adenocarcinoma received an EGFR TKI (either gefitinib or erlotinib). Patients who had received EGFR TKI for less than 2 weeks (n=7), those who did not undergo follow-up chest tomography after EGFR TKI administration (n=8), or those who had other primary cancers (n=3) were excluded from the study. Ultimately, 66 patients were enrolled. Gefitinib or erlotinib was administered orally (250 mg and 150 mg, respectively) once daily until disease progression or unacceptable toxicity.
Demographic and clinical characteristics including age, sex, smoking status, stage at EGFR TKI use, Eastern Cooperative Oncology Group (ECOG) performance status (PS) at EGFR TKI administration, the EGFR TKI agent administered (gefitinib or erlotinib), the number of previous chemotherapy regimens received, and extrathoracic metastatic sites (bones, brain, adrenal gland, liver, lymph nodes, and others locations as evaluated on the TKI administration start date) were retrieved from the medical records.
The study was approved by the institutional review board of Korea Cancer Center Hospital (IRB No. K-1504-001-003) and was conducted in accordance with the Declaration of Helsinki. The patients provided written informed consent to undergo the EGFR mutation and SISH tests.
2. Response assessment
Tumor response was assessed according to the Response Evaluation Criteria in Solid Tumors. The patients underwent chest computed tomography scans 4–8 weeks after initiating of EGFR TKI treatment, and then every 8–12 weeks thereafter. Brain magnetic resonance imaging or radionuclide bone scanning was also performed if brain or bone metastases were suspected, respectively. Patients who had complete response, partial response, or stable disease were categorized as responders; those who had progressed were categorized as non-responders. Progression-free survival (PFS) and overall survival (OS) were calculated from the first date of EGFR TKI treatment to the date of disease progression and the date of death or last follow-up, respectively.
3. EGFR genotyping
Genomic DNA was extracted from paraffin-embedded tissues, as described previously18. In patients whose only available tissue was the cytological sample obtained at initial diagnosis, methanol-fixed cytological specimens were used to extract DNA19. EGFR mutation analysis was performed by pyrosequencing using previously described methods20. The presence of EGFR mutations was determined by evaluating exons 18, 19, 20, and 21.
4. Tissue samples for EGFR SISH
Formalin-fixed paraffin-embedded (FFPE) tumor tissue samples and cell blocks of aspiration specimens were selected from a tissue and cytology archive of samples at the Department of Pathology at Korea Cancer Center Hospital. Hematoxylin and eosin staining was performed to assess sample adequacy and to confirm the histological diagnosis. Thirty-one tissue samples from the 66 patients were available for EGFR SISH analysis.
5. EGFR SISH analysis and evaluation of SISH signals
Dual-color dual-hapten SISH was performed according to the EGFR dinitrophenol (DNP) probe with the chromosome 7 digoxigenin (DIG) probe and ultraVIEW SISH DNP Detection Kit/ultraVIEW RED ISH DIG Detection Kit (Ventana Medical System Inc., Tucson, AZ, USA) for EGFR and chromosome 7 quantitation. The procedure was performed on an automated Ventana Benchmark XT (Ventana/Roche; Ventana Medical System Inc.) according to the manufacturer's standard protocols. Four-micrometer-thick FFPE samples from each case were deparaffinized, denatured, and pretreated with a Tris-based reaction buffer (pH 7.6) in 3 cycles of 12 minutes each at 90℃, and were then incubated with ISH Protease (Ventana/Roche) for 24 minutes. The DNP-labeled EGFR DNA probe and DIG-labeled chromosome 7 probe were co-denatured for 12 minutes at 80℃; slides were hybridized for 7 hours at 44℃ and were then washed stringently 3 times for 8 minutes at 72℃. EGFR signals were detected using the ultraVIEW SISH DNP Detection Kit. The signal was detected as silver deposits with silver acetate, hydroquinone, and hydrogen peroxide. The chromosome 7 signals were detected using the ultraVIEW RED ISH DIG Detection Kit, and were developed as stained red dots with fast red and naphthol phosphate. The slides were finally counterstained with hematoxylin II and bluing reagent before mounting. EGFR SISH scores were defined according to the Colorado scoring system as score 1 (1 or 2 signals in ≥90% of the counted nuclei [disomy]); score 2 (3 signals in 10–40%, ≥4 signals in <10% [low trisomy]); score 3 (3 signals in ≥40%, ≥4 signals in <10% [high trisomy]); score 4 (≥4 signals in 10%–40% [low polysomy]); score 5 (≥4 signals in ≥40% [high polysomy]); and score 6 (amplification [EGFR gene cluster ≥10%, EGFR/chromosome 7 ratio of ≥2, and ≥15 signals in ≥10%])14.
6. Statistical analyses
Categorical variables were analyzed using Fisher exact test, while continuous variables were analyzed using the Mann-Whitney test. The associated factors with treatment response of EGFR TKI were identified by univariate and multivariate logistic regression. PFS and OS were estimated by using the Kaplan-Meier method. Comparisons between groups were performed using the log-rank test for time-to-event variables. Cox proportional hazards models were used to evaluate independent predictive factors for survival. All statistical analyses were performed using SPSS software version 18.0 (SPSS Inc., Chicago, IL, USA), and p-values of <0.05 were considered statistically significant.
Results
1. Patients characteristics
A total of 66 patients who received EGFR TKIs for lung adenocarcinoma with EGFR wild-type were identified for the analyses. The median follow-up time was 17.0 months (range, 1.9–81.3 months). The patients' median age was 57 years, and 66.7% were men (Table 1). The proportion of ever smokers was 69.1%. Approximately half of all patients were treated with EGFR TKIs beyond second-line therapy. Most patients (97.0%) had stage IV disease; 78.8% had extrathoracic metastasis.
The tumor responses to EGFR TKIs are shown in Table 1; the objective response rate was 12.1% while the disease control rate was 40.9%.
2. EGFR SISH and TKI efficacy
We performed EGFR SISH in 31 NSCLC specimens; analysis was possible in 23 of these cases. Disomy was present in one patient (4.3%), low trisomy in three (13.0%), high trisomy in three (13.0%), low polysomy in five (21.7%), high polysomy in 10 (43.5%), and gene amplification in one (4.3%). SISH positivity was defined as high polysomy or gene amplification. Twelve patients (52.2%) were categorized as EGFR SISH positive (high EGFR copy number), and 11 (47.8%) were characterized as EGFR SISH negative (low EGFR copy number). There was no significant difference in clinical characteristics between EGFR SISH-positive and -negative patients (Table 2). There was a trend toward higher response and disease control rates in SISH-positive patients compared to SISH-negative patients (16.7% vs. 0%, p=0.478 and 33.4% vs. 18.2%, p=0.640, respectively). The median PFS and OS were longer in SISH-positive patients than in SISH-negative patients (PFS: 2.0 months, vs. 1.0 month respectively; hazard ratio [HR], 0.57; 95% confidence interval [CI], 0.24–1.35; p=0.201 and OS: 7.5 months vs. 3.0 months, respectively; HR, 0.55; 95% CI, 0.23–1.30; p=0.173), but the differences were not statistically significant.
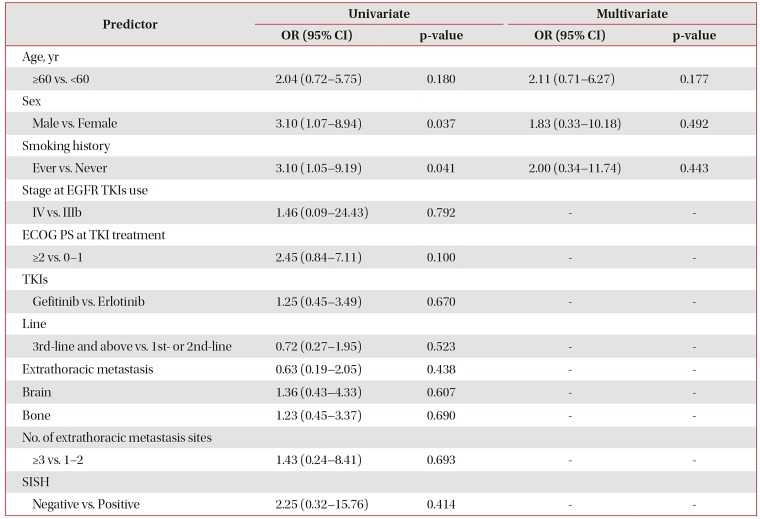
To investigate the baseline clinical variables that could predict treatment response to EGFR TKIs, logistic regression models were used to evaluate the correlation between these factors and treatment response. On univariate analyses (Table 3), female sex and never smoker were significantly associated with better treatment response (odds ratio [OR], 3.10; 95% CI, 1.07–8.94; p=0.037 and OR, 3.10; 95% CI, 1.05–9.19; p=0.041). But, there was no clinical factor to predict treatment response on multivariate analysis.
3. Survival outcomes
The overall median PFS and OS rates of all 66 patients were 2.1 months (95% CI, 1.77–2.43) and 9.7 months (95% CI, 6.35–13.05), respectively. Univariate analysis of PFS showed that female sex and ECOG PS scores of 0–1 had significantly positive effects on PFS; both factors were also significant independent predictors of PFS on multivariate analysis (Table 4). Furthermore, female sex, never smoker status, ECOG PS 0–1, and a low number of extrathoracic metastasis sites were significant predictors of longer OS on univariate analysis (Table 5). Treatment with EGFR TKIs beyond second-line therapy was associated with poor OS. On multivariate analysis, an ECOG PS 0–1 and a low tumor burden of extrathoracic metastases were independent predictors of longer OS.
Discussion
In our study of 66 patients with EGFR wild-type lung adenocarcinoma who received EGFR TKIs, the response and disease control rates were 12.1% and 40.9%, respectively, which are similar to results from previous studies. The First-SIGNAL trial reported a 25.9% response rate and a 40.7% disease control rate to gefitinib in never smokers with EGFR wild-type lung adenocarcinoma21. Another Asian study reported a 21.9% response rate and a 38.4% disease control rate with EGFR TKI22. However, the response rate in patients with EGFR wild-type was only 1.1% in the Iressa Pan-Asia Study (IPASS)4. These discrepant results may be explained by differences in the EGFR mutation detection method. Pyrosequencing was used in our study, whereas the more sensitive amplification-refractory mutation system was used in the IPASS trial; this might account for a relatively higher false-negative rate.
The role of EGFR copy number as a predictive biomarker for the efficacy of EGFR TKIs remains controversial23. Several studies suggested that a high EGFR gene copy number correlated with EGFR TKI treatment response and longer survival in patients with EGFR wild-type lung cancer9101112. In a recent study of patients with EGFR wild-type NSCLC, those who were EGFR FISH-positive achieved a significantly higher response rate (17.7% vs. 8.6%, p=0.047) and disease control rate (67.7% vs. 35.7%, p<0.001), as well as longer PFS (4.4 months vs. 2.0 months; HR, 0.56; 95% CI, 0.41–0.75; p<0.001) and OS (25.0 months vs. 14.2 months; HR, 0.60; 95% CI, 0.41–0.89; p=0.010) than patients who were FISH-negative9.
We showed that EGFR SISH was positive in 12 of 23 patients (52.2%) with EGFR wild-type lung adenocarcinoma. PFS and OS tended to be longer in SISH-positive patients than SISH negative patients. Moreover, the response and disease control rates were higher in SISH-positive patients than in SISH-negative patients. However, there were no significant differences in survival and tumor response between the EGFR SISH-positive and -negative patients. Lee et al.24 showed that a high EGFR gene copy number was associated with better responses to EGFR TKI (27.3% vs. 4.2%, p=0.082) and PFS (4.10 months [95% CI, 1.66–6.54] vs. 2.10 months [95% CI, 1.21–2.99], p=0.201) in patients with EGFR wild-type squamous cell carcinoma; however, these associations did not reach statistical significance. Li et al.25 investigated the efficacy of erlotinib versus pemetrexed as second-line treatments for patients with EGFR wild-type and EGFR FISH-positive lung adenocarcinoma, and found that the median PFS was 4.1 months (95% CI, 1.6–6.6) and 3.9 months (95% CI, 2.7–5.1), respectively, in the erlotinib and pemetrexed arms (HR, 0.92; 95% CI, 0.62–1.37; p=0.693). The objective response rate appeared to be higher among patients receiving erlotinib compared with those receiving pemetrexed (19.7% vs. 8.1%, respectively; p=0.062). Their study did not reveal differences in survival and responses between erlotinib and pemetrexed in patients with EGFR wild-type and EGFR FISH-positive adenocarcinoma.
In our study, good PS and female sex were associated with better PFS. An ECOG PS 0–1 and a low tumor burden of extrathoracic metastases were independent predictors of longer OS. Several studies reported that PS was predictive factor for response to EGFR TKI in patients with wild-type EGFR2627 and female sex was independent good predictors of gefitinib treatment in patients with advanced non-small cell lung cancer2829.
Sanchez de Cos Escuin et al.30 reported that the number of metastatic sites and of lesions in patients with isolated metastasis had prognostic relevance. Tumor burden is a predictive factor for response and survival in patients with NSCLC irrespective of the therapeutic agents employed, including cytotoxic chemotherapy and TKI313233. Higher tumor burdens may reflect the rapid growth and greater number of cancer cells and be associated with poor prognosis.
The present study has several limitations. It was underpowered due to the small sample size, which may have been a factor in the failure to detect differences between EGFR SISH-positive and -negative patients. As ours was a single-center retrospective study, future prospective studies ought to be conducted to validate our findings.
In conclusion, we found that PS, female sex, and tumor burden are predictive factors of the clinical benefit of EGFR TKI therapy in EGFR wild-type lung adenocarcinoma. However, EGFR SISH status was not predictive of EGFR TKI efficacy.
Acknowledgments
This study was supported by a grant of the Korea Institute of Radiological and Medical Sciences, funded by Ministry of Science, ICT and Future Planning, Republic of Korea (1711045543/50474-2017). The authors have no financial conflicts of interest.
Notes
Authors' Contributions:
Conceptualization: Kim CH, Kim SY.
Formal analysis: Kim SY, Myung JK.
Data curation: all authors.
Writing - original draft preparation: Kim SY, Myung JK.
Writing - review and editing: Kim SY, Myung JK.
Approval of final manuscript: all authors.
Conflicts of Interest: No potential conflict of interest relevant to this article was reported.