CT-Guided Percutaneous Transthoracic Needle Biopsy Using the Additional Laser Guidance System by a Pulmonologist with 2 Years of Experience in CT-Guided Percutaneous Transthoracic Needle Biopsy
Article information
Abstract
Background
We developed an additional laser guidance system to improve the efficacy and safety of conventional computed tomography (CT)–guided percutaneous transthoracic needle biopsy (PTNB), and we conducted this study to evaluate the efficacy and safety of our system.
Methods
We retrospectively analyzed the medical records of 244 patients who underwent CT-guided PTNB using our additional laser guidance system from July 1, 2015, to January 20, 2016.
Results
There were nine false-negative results among the 238 total cases. The sensitivity, specificity, positive predictive value, negative predictive value, and diagnostic accuracy of our system for diagnosing malignancy were 94.4% (152/161), 100% (77/77), 100% (152/152), 89.5% (77/86), and 96.2% (229/238), respectively. The results of univariate analysis showed that the risk factors for a false-negative result were male sex (p=0.029), a final diagnosis of malignancy (p=0.033), a lesion in the lower lobe (p=0.035), shorter distance from the skin to the target lesion (p=0.003), and shorter distance from the pleura to the target lesion (p=0.006). The overall complication rate was 30.5% (74/243). Pneumothorax, hemoptysis, and hemothorax occurred in 21.8% (53/243), 9.1% (22/243), and 1.6% (4/243) of cases, respectively.
Conclusion
The additional laser guidance system might be a highly economical and efficient method to improve the diagnostic efficacy and safety of conventional CT-guided PTNB even if performed by inexperienced pulmonologists.
Introduction
Conventional computed tomography (CT)–guided percutaneous transthoracic needle biopsy (PTNB) is a well-established method and is the most common imaging modality used to obtain tissue from a lung lesion1. Cone-beam CT (CBCT)–guided PTNB has recently been in the spotlight because of its real-time fluoroscopic capability, greater flexibility in orientating the detector around the patient, and relatively low radiation exposure compared with CT fluoroscopy-guided PTNB23. The advantage of CBCT-guided PTNB over conventional CT-guided PTNB is its real-time properties that enable operators to avoid dangerous structures and access the target lesion in patients unable to cooperate with breath holding3. Lee et al.3 reported the outcomes of CBCT-guided PTNB in 1,108 patients. The results showed that the sensitivity, specificity, diagnostic accuracy, and incidence of complications (pneumothorax and hemoptysis) were 95.7% (733/766), 100% (323/323), 97.0% (1,056/1,089), and 23.9% (276/1,153), respectively. However, several studies on conventional CT-guided PTNB involving more than 100 patients have been reported since 2010, all of which showed similar results to those of CBCT-guided PTNB (sensitivities, 90%–96%; specificities, 92%–100%; diagnostic accuracies, 93%–97%)567891011. Choi et al.11 performed conventional CT-guided PTNB on patients with pulmonary nodules <1 cm and reported a sensitivity, specificity, and diagnostic accuracy of 93.1% (148/159), 98.8% (81/82), and 95.0% (229/241), respectively. These results are comparable with those of a study that performed CBCT on pulmonary nodules ≤1 cm, in which the sensitivity, specificity, and diagnostic accuracy were 96.7% (58/60), 100% (38/38), and 98.0% (96/98), respectively12. CBCT is very expensive and requires a large physical space for the equipment. Therefore, considering the diagnostic yield and patient safety of conventional CT reported in recent studies, which are comparable with those of CBCT, it is preferable to improve existing conventional CT-guided PTNB than to purchase a CBCT system12341112. We developed the additional laser guidance system to improve the efficacy and safety of conventional CT-guided PTNB. This study was conducted to evaluate the efficacy and safety of our laser guidance system.
Materials and methods
1. Patients
We performed a retrospective analysis of the medical records of 244 patients who underwent PTNB from July 1, 2015, to January 20, 2016. Approval for this study was given by the Chungnam National University Hospital Institutional Review Board (CNUH 2016-02-004), and requirement for informed consent was waived.
2. Procedures
1) Installation and alignment of the additional laser guidance system
The installation and alignment of the additional laser guidance system were as follows. (1) We mounted the laser level (HG-909A; X-CLOVE, China), which adhered to a wheel bracket, on a stainless steel frame and positioned it on the opposite side of the CT gantry (Figures 1, 2A). (2) By adjusting the wheel bracket (to the left or right), we matched both vertical laser beam lines, which came from the laser level (HG-909A; X-CLOVE) and the CT gantry (Figure 2B). (3) The second laser level, also mounted on an iron frame, was placed on the opposite side of the patient to the operator, perpendicular to the other laser (Figure 2C).
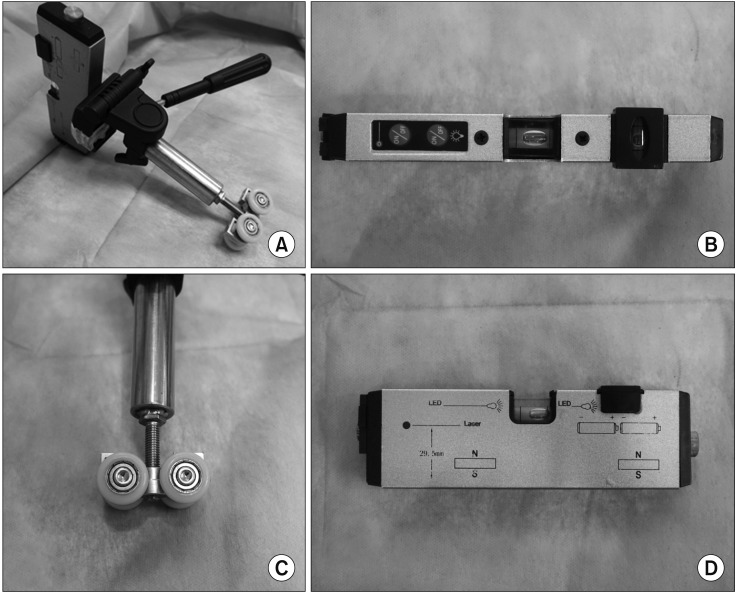
Laser level (HG-909A; X-CLOVE) and a wheel bracket. (A) Laser level, adhered to a wheel bracket. (B, D) Overhead and side view of laser level. (C) Wheel part of a bracket.

(A) The laser level, adhered to a wheel bracket, mounted on a stainless steel frame and positioned on the opposite side of the computed tomography (CT) gantry. Horizontal movement is possible (blue arrows). (B) Both vertical laser beam lines, which came from the laser level and CT gantry, had been aligned, such that they looked like a line (red arrows). (C) The second laser level was placed on the side of the patient opposite to the operator, perpendicular to the other laser. (D) In the case of an oblique approach, a digital level (DWL-80E, Sincon) was used to tilt the laser level. The digital level was 29.9° (red round arrows).
2) CT-guided PTNB procedure
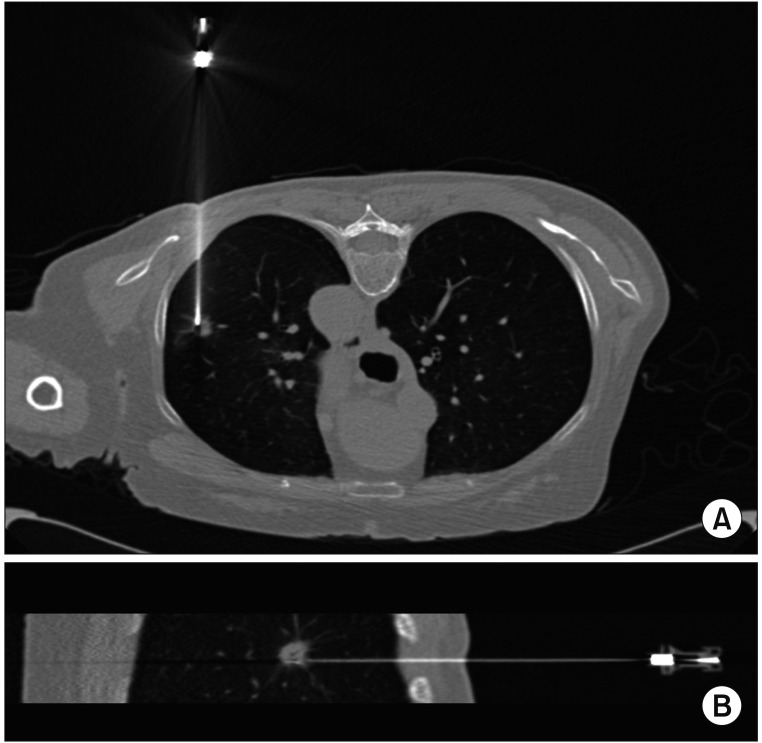
All PTNBs were performed under CT (Siemens SOMATOM Sensation 64 CT scanner; Siemens Medical Solutions, Forchheim, Germany) guidance by one pulmonologist (D.I. Park with 2 years of experience in CT-guided PTNB). Before CT was initiated, the importance of breathing was explained to each patient, who was asked to practice breathing in at tidal volume and holding it three times. After obtaining a CT scout image, low-dose (100 kVp, 20 mA) CT was performed at the area of interest using a 2-mm section thickness and with the patient practicing breath-holding after inspiration. The subsequent procedures were as follows. (1) The skin entry site was determined by the CT gantry laser lights and radiopaque grid displayed on the skin and was sterilized using Betadine. (2) Local anesthesia from the skin to the pleural surfaces was administered using a 1% lidocaine solution. (3) By adjusting the laser position when the patient inhaled and held their breath, the intersection of the two laser lines originating from the head end and the side opposite to the operator was located at the entry point on the skin (Figure 3). In the case of an oblique approach, we used a digital level (DWL-80E; Sincon, China) to tilt the laser level (HG-909A; X-CLOVE) before performing procedure (Figure 2D). (4) A coaxial needle was inserted along the two laser lines into the target lesion via the skin entry site (Figure 3D). (5) A CT scan was conducted to ensure that the coaxial needle tip was in the correct position (Figure 4). (6) Then, a semi-automatic cutting needle ranging in size from 20 to 16 gauge (Stericut; TSK Laboratory, Tochigi, Japan) was inserted via the coaxial introducer, and the biopsy was performed. Specimen acquisition was repeated until a visually adequate amount of tissue was obtained. To check for biopsy-related complications, CT was performed after obtaining the tissue.
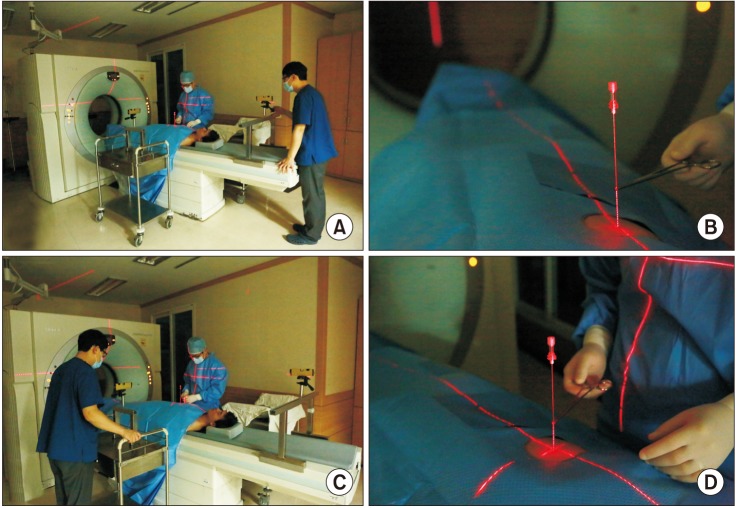
Adjustment of the laser lines. (A, B) The laser line originating from the head end was located at the entry point on the skin and ran along the coaxial needle. (C, D) The intersection of the two laser lines originating from the head end and the side opposite to the operator were matched at the entry point on the skin and ran long the coaxial needle.
3. Statistical analysis
To determine the risk factors for diagnostic failure, the study population was divided into a diagnostic success group (i.e., true-positive and true-negative results) and a diagnostic failure group (i.e., false-positive and false-negative results). The factors related to diagnostic failure were evaluated by univariate analyses using the Mann-Whitney U test for numerical values and the chi-square test for categorical values. Statistical analysis was performed using PASW Statistics version 18.0 (IBM Corp., Armonk, NY, USA). A p-value of < 0.05 was considered to indicate statistical significance.
Results
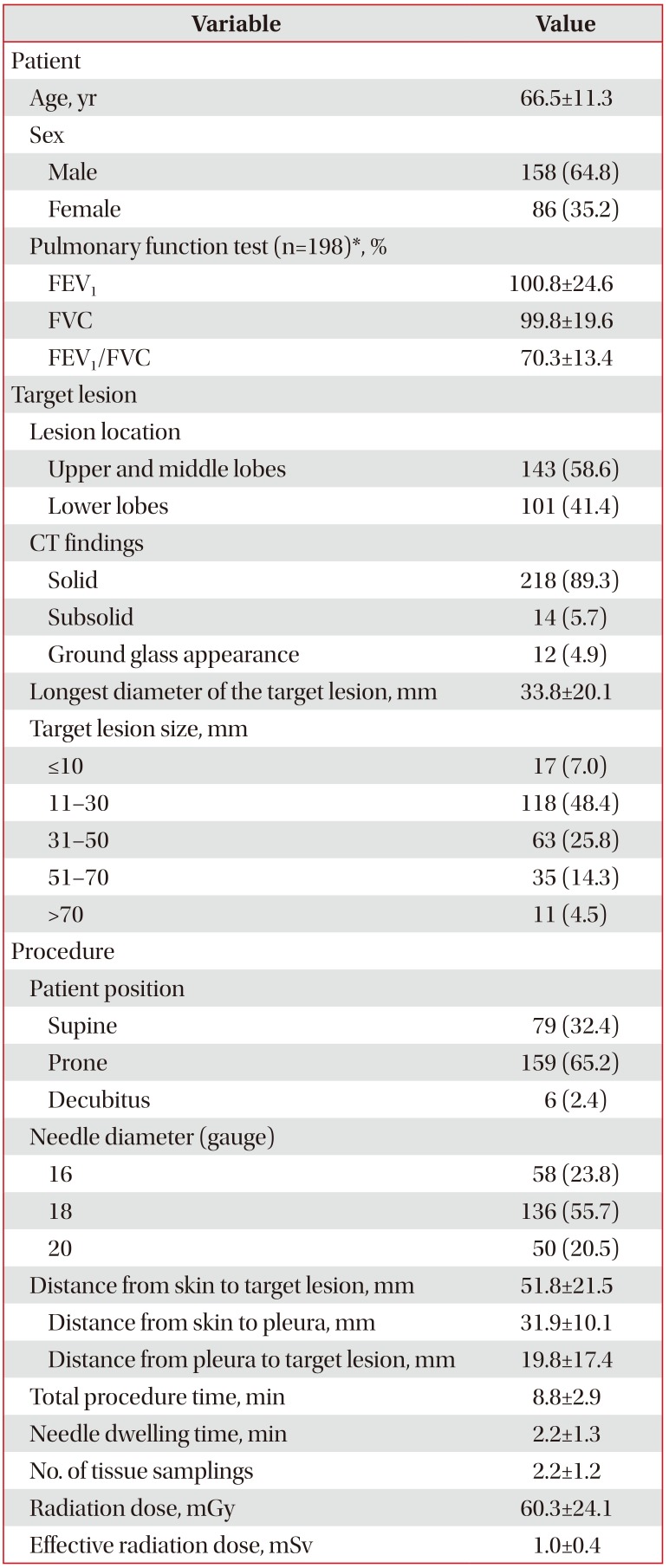
1. Patient and target lesion characteristics
The baseline patient characteristics and procedures performed are summarized in Table 1 (n=244). The mean age of the patients was 66.5±11.3 years, and 64.8% (n=158) of all cases were male. Among all target lesions, 41.4% (n=101) were located in the lower lobes. The mean longest diameter was 33.8±20.1 mm. The numbers of solid, sub-solid, and groundglass lesions were 218 (89.3%), 14 (5.7%), and 12 (4.9%), respectively.
2. Procedure and radiation dose
During the procedure, 79 (32.4%) patients were placed in the supine position, 159 (65.2%) in the prone position, and six (2.4%) in the decubitus position; 58 (23.8%) 16-gauge biopsy needles, 136 (55.7%) 18-gauge needles, and 50 (20.5%) 20-gauge needles were used. The mean distance from the skin to the target lesion was 51.8±21.5 mm. The total procedure time was 8.8±2.9 minutes, and the needle dwelling time was 2.2±1.3 minutes. The number of biopsies performed was 2.2±1.2. The calculated mean effective radiation dose was 1.0±0.4 mSv.
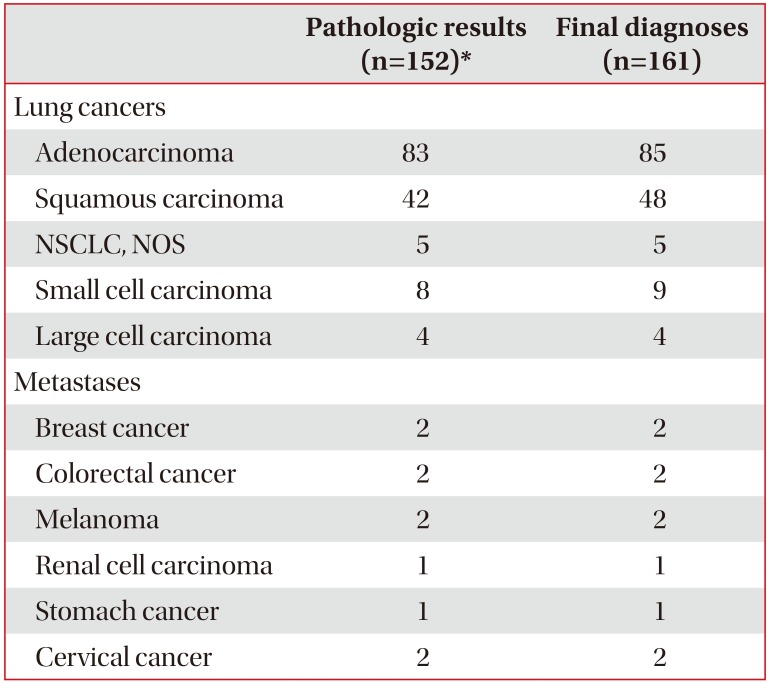
3. Pathological results and final diagnoses
A lung lesion was recorded as ‘benign’ if the nodule met the following criteria. (1) The pathologic results showed definitive benign features (e.g., aspergilloma, tuberculosis). (2) The pathologic results did not show definitive benign features (e.g., inflammation, fibrosis); for example, (a) the CT findings favored a benign lesion, (b) the size of the lesion decreased or showed no interval change on follow-up CT after more than 18 months, or (c) the physician decided to discontinue follow-up because the lesion was regarded as benign. (3) The pathologic results confirmed the nodule as “benign” on more than two occasions. All lesions not determined to be benign or malignant were classified as “indeterminate” and were excluded from the statistical analysis.
Among the technically successful cases (n=243), 161 (66.3%), 77 (31.7%), and five (2.1%) were diagnosed as malignant, benign, and indeterminate lesions, respectively. Of the malignant lesions (n=161), 152 (94.4%) were diagnosed by PTNB. False-negative cases (n=9) were finally diagnosed as malignancy by re-PTNB (n=4), bronchoscopy (n=2), surgical biopsy (n=1), endobronchial ultrasound (n=1), and pleural effusion cytology (n=1). Of the 77 (31.7%) lesions diagnosed as benign, 32 (13.2%) had specific benign pathologic features, with the most common benign lesion being tuberculosis (n=16, 6.6%). There were five indeterminate cases. In the remaining technically successful cases, excluding the indeterminate cases, the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and diagnostic accuracy for the diagnosis of malignancy were 94.4% (152/161), 100% (77/77), 100% (152/152), 89.5% (77/86), and 96.2% (229/238), respectively. The pathologic results and final diagnoses of the malignant lesions are summarized in Table 2.
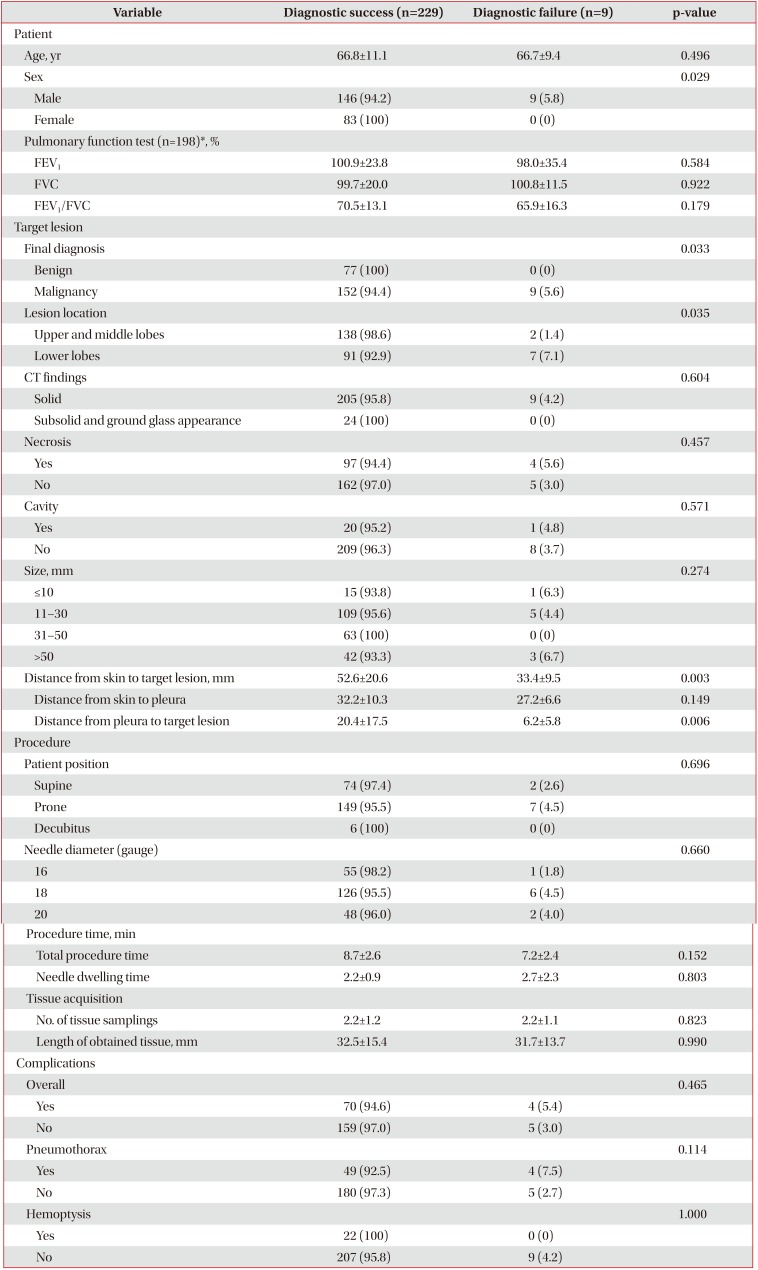
4. Risk factors for diagnostic failure
There were nine false-negative results among the 238 total cases. The results of the univariate analyses showed that the risk factors for a false-negative result were male sex (p=0.029), a final diagnosis of malignancy (p=0.033), a lesion in the lower lobe (p=0.035), and a shorter distance from the pleura to the target lesion (p=0.006). The longest diameter of the target lesion was not related to diagnostic failure (p=0.274) (Table 3).
5. Complications and management
The overall complication rate was 30.5% (74/243). Pneumothorax, hemoptysis, and hemothorax occurred in 21.8% (53/243), 9.1% (22/243), and 1.6% (4/243) of cases, respectively. In terms of pneumothorax management, aspiration by syringe (3.7%, 9/243) and chest tube drainage (5.3%, 13/243) were performed. All hemoptysis (n=22) and hemothorax (n=4) cases recovered after oxygen therapy and close observation.
Discussion
In this study, we performed CT-guided PTNB using the additional laser guidance system. Our additional laser guidance system facilitated the ideal trajectory angle of the coaxial needle though the skin and pleura. The results showed that the sensitivity, specificity, PPV, NPV, and diagnostic accuracy for the diagnosis of malignancy were 94.4% (152/161), 100% (77/77), 100% (152/152), 89.5% (77/86), and 96.2% (229/238), respectively.
These results are comparable with the outcomes2 of the initial experience with CBCT-guided PTNB, performed in 71 patients with lung nodules ≤30 mm. The sensitivity, specificity, diagnostic accuracy, and incidence of complications in that study were 97% (35/36), 100% (25/25), 98.4% (60/61), and 38.0%, respectively.
In terms radiation exposure, the mean effective radiation dose in our study was 1.0±0.4 mSv. This is less than the 7.3 mSv and 4.6 mSv doses of CBCT reported by Lee et al.3 and Hwang et al.13, respectively, and is similar to the mean effective dose of the National Lung Screening Trial (1.4±0.5 mSv)14. The additional laser guidance system provides operators guidance and confidence regarding the correct insertion angle of the coaxial needle, thereby decreasing the time and number of adjustments to the needle location required to approach the target lesion. We believe that these factors can reduce radiation exposure.
Regarding diagnostic failure, there was one technical failure case and nine false-negative cases. The one technical failure was caused by a marking error (marking the left lung lesion as the right lung lesion) made by the radiologic technologist when determining the skin entry site, and this case was not included in the analysis of the diagnostic yield. In this study, male sex (p=0.029), a final diagnosis of malignancy (p=0.033), a lesion in the lower lobe (p=0.035), a shorter distance from the skin to the target lesion (p=0.003), and a shorter distance from the pleura to the target lesion (p=0.006) were significantly related to false-negative results. Our finding that a malignant final diagnosis was a risk factor for diagnostic failure corresponds to the results of previous studies815. A lower lobe lesion is a well-known risk factor for diagnostic failure and is explained by the greater respiratory motion of the lower lobe during the procedure3815. In several studies, male sex has been reported as a risk factor for pneumothorax and for diagnostic failure3816. Known patient-related risk factors for pneumothorax, such as chronic obstructive pulmonary disease, emphysema, and a history of smoking, are more common in males1718.
Interestingly, our results showed that shorter distances from the pleura to the target lesion (p=0.006) were significantly related to diagnostic failure. In studies analyzing the relationship between the distance from the pleura to the target lesion and diagnostic failure, there was a tendency toward increased diagnostic failure if the lesion was too close or too distant from the pleura31519. Ko et al.20 reported that adjacent pleural disease is a risk factor for pneumothorax. Other studies found that the incidence of pneumothorax increases with decreasing distance between the lesion and pleura321. Biopsy of lesions adjacent to the pleura is technically challenging, because it is very difficult to reposition the needle tip. We believe that pneumothorax and technical difficulty may have influenced our results.
In terms of complications, the rates of overall complications (30.5%, 74/243), pneumothorax (21.8%, 53/243), and hemoptysis (9.1%, 22/243) in the current study were comparable with those of other studies123. The incidence of chest tube placement for pneumothorax was 5.3% (13/243).
There are several disadvantages of CBCT-guided PTNB compared with conventional CT-guided PTNB. First, the operator is exposed to radiation in CBCT-guided PTNB but not in conventional CT-guided PTNB. The radiation exposure to patients is much higher in CBCT-guided PTNB than in conventional CT-guided PTNB, especially when using the low-dose CT protocol2223. Second, the image quality of CBCT is inferior to that of conventional CT2425. In general, reduced image quality does not negatively affect patient safety or the accuracy of PTNB2326. However, if the target lesion is adjacent to the mediastinum or main vessels or is surrounded by pleural effusion, an inferior resolution could affect patient safety and the diagnostic yield. Third, CBCT is very expensive and requires a large physical space for the equipment27. Our laser guidance system, however, is extremely economical and requires approximately 200 USD to build.
One of the limitations of this study was its retrospective design, which is associated with unknown bias. The efficacy of our laser guidance system could have been underestimated as a result of patient selection bias, which is a crucial factor for PTNB results, especially considering that the operator was a pulmonologist with only 2 years of experience. Recently, we conducted a review of medical records to identify rejection cases from October 2016 to May 2017. Surprisingly, there were only seven out of 298 PTNB cases that were rejected for any reason. Another limitation was that we did not compare our method with that of conventional CT-guided PTNB or CBCT-guided PTNB directly. Prospective randomized controlled trials with specific enrollment criteria are needed to determine the efficacy and safety of our method.
In conclusion, the additional laser guidance system might be a highly economical and efficient method to improve the diagnostic efficacy and safety of conventional CT-guided PTNB even performed by a pulmonologist (2 years of experience) who is unexperienced.
Notes
Authors' Contributions: Conceptualization: Park DI, Jeon MC. Methodology: Park DI, Jeon MC. Formal analysis: Park DI, Jeon MC. Data curation: Park DI, Jeon MC. Software: Park DI, Jeon MC. Validation: Kim JO, Jung SS, Park HS, Lee JE, Moon JY, Chung CU, Kang DH. Writing - original draft preparation: Park DI, Jeon MC. Writing - review and editing: Kim JO, Jung SS, Park HS, Lee JE, Moon JY, Chung CU, Kang DH. Approval of final manuscript: all authors.
Conflicts of Interest: No potential conflict of interest relevant to this article was reported.