 |
 |
| Tuberc Respir Dis > Volume 81(2); 2018 > Article |
|
Abstract
Background
Recent studies show that mitophagy, the autophagy-dependent turnover of mitochondria, mediates pulmonary epithelial cell death in response to cigarette smoke extract (CSE) exposure and contributes to the development of emphysema in vivo during chronic cigarette smoke (CS) exposure, although the underlying mechanisms remain unclear.
Methods
In this study, we investigated the role of mitophagy in the regulation of CSE-exposed lung bronchial epithelial cell (Beas-2B) death. We also investigated the role of a phosphodiesterase 4 inhibitor, roflumilast, in CSE-induced mitophagy-dependent cell death.
Results
Our results demonstrated that CSE induces mitophagy in Beas-2B cells through mitochondrial dysfunction and increased the expression levels of the mitophagy regulator protein, PTEN-induced putative kinase-1 (PINK1), and the mitochondrial fission protein, dynamin-1-like protein (DRP1). CSE-induced epithelial cell death was significantly increased in Beas-2B cells exposed to CSE but was decreased by small interfering RNA-dependent knockdown of DRP1. Treatment with roflumilast in Beas-2B cells inhibited CSE-induced mitochondrial dysfunction and mitophagy by inhibiting the expression of phospho-DRP1 and -PINK1. Roflumilast protected against cell death and increased cell viability, as determined by the lactate dehydrogenase release test and the MTT assay, respectively, in Beas-2B cells exposed to CSE.
Chronic obstructive pulmonary disease (COPD) is the fourth leading cause of death worldwide, with cigarette smoke (CS) identified as the main causative agent1. COPD is characterized by progressive obstruction of the airways associated with chronic inflammation of the peripheral airways and progressive destruction of the lung parenchyma2, which contribute to the two major phenotypes of chronic bronchitis and emphysema, each with a different pathophysiology and symptoms3. Chronic inflammation and increased oxidative stress may cause tissue destruction and impair lung maintenance and repair, which are associated with the pathogenesis of emphysema.
It is well known that mechanisms of programmed cell death (i.e., extrinsic and intrinsic apoptosis) contribute to the loss of lung structural cells in the pathogenesis of emphysema4. We and other researchers previously demonstrated the activation of apoptosis signaling pathways in endothelial, epithelial, and fibroblast cells subjected to cigarette smoke extract (CSE) exposure5,6,7. However, the molecular mechanisms of apoptosis and their significance to the etiology of emphysema remain only partially understood.
Recent studies using in vitro and in vivo models of CSE and CS exposure, respectively, as well as human lung tissue from COPD patients, have demonstrated a role for the cellular autophagy pathway (also called macroautophagy) in the pathogenesis of emphysema8,9. Autophagy is a homeostatic process for the turnover of cytoplasmic proteins and organelles. Lung tissue derived from COPD patients or from mice chronically exposed to CS display increased expression levels of autophagy- related proteins and increased autophagosome numbers. A genetic deletion study of crucial autophagy proteins, such as Beclin 1 and microtubule-associated protein-1 light chain-3B (LC3B), revealed the inhibition of CSE-induced lung epithelial cell death in response to CS exposure8.
In addition to the general autophagy pathway, selective forms of autophagy may also be important in the pathogenic mechanisms of COPD. Of these, mitophagy provides a mechanism for the selective autophagic degradation of mitochondria8. According to previous reports, CSE caused mitochondrial dysfunction by decreasing the mitochondrial membrane potential (Δψm) and increasing the production of mitochondrial reactive oxygen species (mtROS). Furthermore, a genetic deficiency experiment of the mitophagy regulator protein, PTEN-induced putative kinase-1 (PINK1), and treatment with the mitophagy/fission inhibitor, Mdivi-1, demonstrated protection against CSE-induced necroptosis and mitochondrial dysfunction in epithelial cells. In lung tissue of COPD patients, lung epithelial cells displayed increased expression levels of PINK1 and the necroptosis protein, receptor-interacting serine/threonine protein kinase 3. These studies have also indicated mitophagy-dependent necroptosis in lung emphysematous changes in response to CS exposure9 and suggest that the activation of mitophagy by CS exposure may promote the induction of necroptosis and lead to depletion of the functional mitochondrial pool during chronic CS exposure9,10,11. Therefore, strategies targeting this pathway may lead to novel therapies for COPD and emphysema. However, the precise mechanism by which mitophagy contributes to lung injury and cell death in the CS exposure model remains unclear. Further studies are needed to improve our understanding of the role of mitophagy in the pathogenesis of emphysema.
Phosphodiesterase-4 (PDE4) inhibitors provide therapeutic benefits, particularly in patients with late-stage inflammation-dominant COPD. Phosphodiesterases (PDEs) are cyclic adenosine monophosphate (cAMP)- or cyclic guanosine monophosphate-specific enzymes that are ubiquitously distributed in most human cells. Eleven PDE isozymes have been identified to date12. Of these, PDE4, which hydrolyzes cAMP, is expressed in all lung structural cells, such as smooth muscle cells, airway epithelium, and inflammatory cells (i.e., neutrophils, lymphocytes, and macrophages)13. Phase III clinical studies have shown that the PDE4 inhibitor roflumilast significantly improved clinical outcomes, such as post-bronchodilator forced expiratory volume in 1 second, and reduced the exacerbation rate and dyspnea severity in the clinical phenotype of chronic bronchitis14. In vivo studies have confirmed that the PDE4 inhibitor roflumilast mitigates emphysema in chronic CS-exposed mice15. In the current study, we examined the functional significance of mitophagy and its relationship with cell death in the context of CSE-induced lung epithelial cell injury. We also investigated the potential therapeutic effects of roflumilast on mitophagy-dependent cell death in CSE-induced Beas-2B cells.
The MitoSOX Red Mitochondrial Superoxide Indicator Kit was purchased from Life Technologies (Carlsbad, CA, USA). The TMRE Mitochondrial Membrane Potential Assay Kit was obtained from Abcam (Cambridge, UK). Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS), and penicillin-streptomycin solution were obtained from Welgene (Gyeongsan, Korea). Dimethyl sulfoxide (DMSO) and 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT) were purchased from Sigma-Aldrich (St. Louis, MO, USA). The following primary antibodies used in this study were purchased from Cell Signaling Technology (Beverly, MA, USA): anti-dynamin-1-like protein (DRP1), anti-phospho-DRP1, anti-PINK1, and anti-actin. The secondary antibodies, anti-rabbithorseradish peroxidase (HRP), and anti-mouse-HRP, were purchased from Santa Cruz Biotechnology (Dallas, TX, USA).
Beas-2B human lung epithelial cells were purchased from Lonza Biologics (Cambridge, UK). Cells were cultured at 37℃ in 5% CO2 in DMEM (Welgene) supplemented with 100 U/mL penicillin, 100 µg/mL streptomycin, and 10% FBS (Welgene). Cells were also treated with 0.01% DMSO as a vehicle control. In the indicated experiments, cells were pre-treated with roflumilast (Santa Cruz Biotechnology) for 2 hours and exposed to 10% CSE for the indicated time intervals.
Kentucky 3R4F research-reference filtered cigarettes (The Tobacco Research Institute, University of Kentucky, Lexington, KY, USA) were consumed using a peristaltic pump (VWR International, Radnor, PA, USA). The filters were removed from the cigarettes before the experiments. Each cigarette was consumed to a 17-mm butt remaining for 7-8 minutes. The smoke from eight cigarettes was bubbled through 40 mL of cell culture medium, and this solution, regarded as 100% strength CSE, was adjusted to a pH of 7.45 and used within 15 minutes of preparation.
Lactate dehydrogenase (LDH) release was measured using a cytotoxicity detection kit (Roche, Indianapolis, IN, USA) according to the manufacturer's protocol. After gentle agitation, 100 µL of culture medium was collected at various times and after different doses during the assay. Cell viability was measured using blue formazan, which is metabolized from MTT by mitochondrial dehydrogenases that are active only in living cells. Beas-2B cells were seeded onto 48-well plates (1×104 cells/well) and cultured in DMEM for the indicated time periods. Cells were exposed to 10% CSE for the indicated time intervals. MTT reagent (5 mg/mL) was added to each well, and the plate was incubated for an additional 2 hours at 37℃. The medium was then removed and the intracellular formazan product was dissolved in 300 µL DMSO. The absorbance of each well was measured at 570 nm on a microplate reader (Molecular Devices Emax, Sunnyvale, CA, USA). Optical density values from untreated control cells were designated as 100%.
mtROS generation was assayed using MitoSOX Red (510/580 nm ex/em ), a mitochondrial superoxide indicator. For the assay, a stock solution of MitoSOX Red was diluted in phosphate-buffered saline (PBS) and added to 10×106 cells/well at a final concentration of 5 µM, and incubated for 10 minutes at 37℃ as described by the manufacturer. Samples were then centrifuged for 5 minutes at 600 ×g, and the pellets were resuspended in 1 mL PBS and subsequently transferred to 5-mL fluorescence-activated cell sorting (FACS) tubes. The TMRE Mitochondrial Membrane Potential Kit uses tetramethylrhodamine, ethyl ester (TMRE; 488/575 nm ex/em) to label active mitochondria. TMRE was added to cells in media at a final concentration of 200 nM and incubated for 20 minutes at 37℃. Samples were then centrifuged for 5 minutes at 600 ×g, and the pellets were suspended in 0.2% bovine serum albumin in PBS and subsequently transferred to 5-mL FACS tubes. For the determination of mtROS and mitochondrial membrane potential (Δψm), the assays were performed on a FACScalibur system (BD Biosciences, San Jose, CA, USA).
mtROS levels and mitochondrial membrane potential in CSE-induced Beas-2B cells were measured using MitoSOX Red and TMRE assays as described by the manufacturer. Briefly, cells were incubated in growth medium containing MitoSOX Red at a final concentration of 5 µM for 10 minutes at 37℃, protected from light. Cells were gently washed one time with warm PBS, and the slides were mounted using 4',6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Burlingame, CA, USA). For each designated treatment, cells were incubated in culture medium with TMRE at a final concentration of 100 nM for 20 minutes at 37℃, protected from light. Cells were gently washed one time with warm PBS, and the slides were mounted using DAPI. The fluorescence of MitoSOX Red and TMRE was detected under a confocal microscope. Digital images were acquired on an inverted confocal laser-scanning microscope (LSM Pascal, Zeiss, Germany) at 2,048×2,048 pixels. Images were captured using either a 40× or 100× oil immersion objective lens, and the optical section was <1 µm.
Proteins were extracted from Beas-2B cells using radioim-munoprecipitation assay buffer (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS], and 1% NP-40) according to the manufacturer's protocol. Then, 20-40 µg of protein from each sample was separated by 10%-15% SDS--polyacrylamide gel electrophoresis and transferred to a polyvinylidene fluoride membrane (Millipore, Bedford, MA, USA). The membrane was blocked with 5% non-fat skim milk (w/v) for 60 minutes at room temperature and incubated with the indicated primary antibody (anti-cleaved-caspase-3, anti-cleaved-caspas-8, anti-DRP1, anti-phospho-DRP1, anti-PINK1, or anti-β-actin) at 4℃ overnight. The membrane was incubated with a 1:3,000-1:5,000 dilution of HRP-conjugated secondary antibody for 1 hour at room temperature. Each protein was detected using the Western Blot Hyper HRP Substrate Kit (TaKaRa Bio, Shiga, Japan). Band intensity was quantified by densitometric analysis using NIH ImageJ software.
Anti-human small interfering RNAs (siRNAs) purchased from Bioneer (Daejeon, Korea) were used for DRP1 and PINK1 knockdown as described by the manufacturer. The target sequences for si-DRP1 included 1040816, 1040813, and 1040810, and those for si-PINK1 included 1116919, 1116915, and 1116923. The latter had the highest inhibition efficiency. Synthetic sequence-scrambled siRNA was used as a negative control (non-targeting) siRNA. Cells were plated and cultured in growth media until cell confluency reached 70%-80% prior to siRNA transfection using Lipofectamine 2000 (Invitrogen, Shanghai, China) according to the manufacturer's instructions. Cells were harvested after 48 hours for Western blot analysis.
To investigate the effect of CSE on mitochondrial function in human bronchial epithelial cells (Beas-2B), we evaluated the mitochondrial membrane potential (Δψm) and mtROS production using TMRE and MitoSOX assays, respectively. CSE caused mitochondrial depolarization and a decrease in functional mitochondria in Beas-2B cells (Figure 1A). We also observed that CSE treatment increased mtROS production in Beas-2B cells in a dose-dependent manner (Figure 1A). To investigate whether CSE induces mitophagy in lung epithelial cells, we measured the expression of p-DRP1 and PINK1, regulator proteins of mitophagy. Compared to untreated cells, the expression of p-DRP1 and PINK1 was increased by 16.4- and 5.7-fold, respectively, in Beas-2B cells after CSE treatment (Figure 1B). These results suggest that CSE induces mitophagy in lung bronchial epithelial cells, which is associated with increased expression of mitophagy-related proteins. After CSE exposure, p-DRP1 and PINK1 expression was preferentially increased in the mitochondrial fraction of Beas-2B cells (Figure 1C).
As shown in Figure 2A and B, cell viability, as determined by the MTT assay, and cell death, as determined by the LDH release assay, were effected in a dose-dependent manner after 18 hours of CSE exposure. To determine whether DRP1 and/or PINK1 play a role in CSE-induced cell death, DRP1 or PINK1 was knocked down in Beas-2B cells using DRP1-or PINK1-targeted siRNA. As shown in Figure 2C, control siRNA-transfected cells displayed increased LDH release after CSE treatment (5.71-fold relative to untreated cells), whereas knockdown of DRP1 or PINK1 in Beas-2B cells resulted in decreased LDH release after CSE treatment (3.42- and 3.55-fold, respectively) relative to untreated cells. Moreover, the MTT assay revealed that knockdown of DRP1 decreased cellular cytotoxicity following CSE exposure compared to control siRNA-infected cells, whereas knockdown of PINK1 did not affect cell viability (Figure 2D).
Next, we investigated the role of roflumilast in mitochondrial dysfunction and mitophagy induced by CSE exposure using confocal microscopy and Western blot analysis. Roflumilast pretreatment markedly protected against mitochondrial dysfunction, as evident by an increase in functional mitochondria and a decrease in mtROS production in CSE-treated cells compared with that observed in epithelial cells treated with CSE only (Figure 3A). Roflumilast also decreased the expression of mitophagy regulator proteins, such as p-DRP1 and PINK1 (Figure 3B).
Roflumilast markedly decreased CSE-induced cytotoxicity, as measured by the LDH release assay, and increased cell viability, as assessed by the MTT assay (Figure 4A, B). To explore the role of roflumilast in CSE-induced apoptotic cell death, we examined the effect of roflumilast on known apoptosis-related proteins, such as cleaved caspase-3 and -8. Treatment with roflumilast markedly inhibited the expression of these proteins (Figure 3C). The efficacy of roflumilast against CSE-induced cell cytotoxicity was apparent at the concentration of 5 µM roflumilast. DRP1 siRNA-transfected cells showed no more protection following roflumilast treatment in CSE-induced cell death; however, PINK1 siRNA-transfected cells exhibited increased protection following the addition of roflumilast (Figure 4C).
Traditionally, chronic inflammation, increased oxidative stress, and/or impaired lung repair processes have been known to contribute to the pathogenesis of emphysema, which is mainly caused by CS and biomass equivalent, and is characterized by an increase in alveolar wall cell death and/or a failure of alveolar wall maintenance. There are no current therapies available that either prevent or cure the progression of emphysema16,17. Preclinical studies have shown that PDE4 inhibitors consistently reduce the accumulation of neutrophils in bronchoalveolar lavage fluid following short-term exposure to CS in mice 18. Thus, PDE4 inhibitors have been exploited primarily for their anti-inflammatory effects in the treatment of COPD, especially in the chronic bronchitis-dominant phenotype. However, several studies that investigated the effects of PDE4 inhibitors on the development of emphysema19 have mainly focused on apoptosis20. We also previously investigated the effects of a PDE4 inhibitor in CSE-induced apoptosis of lung structural cells, and revealed that the PDE4 inhibitor rolipram protects lung fibroblasts from CSE-induced apoptosis by inhibiting the activation of caspase-3 and -821. Autophagy, a cellular pathway for the degradation of damaged organelles and proteins, has gained increasing importance in human diseases, both as a modulator of pathogenesis and as a potential therapeutic target22,23. In addition, autophagy can influence most fundamental processes, including inflammation and immune responses, the host defense, metabolic pathways, and programmed cell death. Some studies have suggested that autophagy promotes lung epithelial cell death, airway dysfunction, and ultimately emphysema in response to CS exposure9,24,25,26. Furthermore, recent studies have reported that CS exposure in epithelial cells promotes the autophagy-dependent turnover of mitochondria (mitophagy) via mitochondrial dysfunction, as evident by a decrease in mitochondrial membrane potential and an increase in mtROS production9. Here, we confirmed that CSE causes mitochondrial dysfunction using TMRE and MitoSOX assays. We also demonstrated that roflumilast markedly inhibited mitochondrial dysfunction by increasing functional mitochondria and decreasing mtROS production.
In general, mitophagy is a cellular response that is commonly associated with mitochondrial dysfunction8. PINK1 and Parkin represent well-recognized regulators of mitophagy in neural cells and tissues27. There are two altered mitochondrial dynamics in terms of mitophagy (i.e., fusion or fission), which play key roles in the response of cells to exogenous stress. Mitochondrial fission, a process regulated by DRP1, is needed to trigger mitophagy28. The phosphorylation of DRP1 promotes DRP1 recruitment to mitochondria29. In adult cardiac myocytes, overexpression of the dominant-negative form of DRP1 results in decreased mitochondrial fission and mitophagy29. Mild oxidative stress specifically triggers mitophagy in a DRP1-dependent manner11. Recent studies have applied Mdivi-1, a pharmacological inhibitor of DRP1, for the functional study of mitophagy30,31,32,33. We confirmed that CSE increased the expression of mitophagy-associated proteins in Beas-2B cells. Furthermore, we showed that the expression levels of p-DRP1 and PINK1 induced by CSE exposure were significantly reduced after pre-treatment with roflumilast. And the MTT assay demonstrated that knockdown of DRP1 decreased cellular cytotoxicity following CSE exposure compared to control siRNA-infected cells, whereas knockdown of PINK1 did not affect cell viability. These inconsistent results of PINK1 need to be elucidated although generally PINK1 promotes cell death in response to CSE.
Many studies have implicated CS-induced apoptosis in alveolar epithelial cells as a possible mechanism for the development of emphysema21,34. CS can induce apoptosis and the production of apoptogenic factors in various structural lung cell types, including alveolar macrophages, bronchial epithelial cells, endothelial cells, and fibroblasts35. In vivo, chronic CS exposure of mice resulted in a significant increase in apoptotic cells in the bronchial and alveolar epithelia36. We previously reported that CSE induced Fas receptor-mediated death-inducing signaling complex formation, the most proximal event in the extrinsic apoptotic pathway6. CSE has been shown to induce both apoptosis and necrotic cell death in airway epithelial cells37. We also found that roflumilast treatment protected against the decrease in viable cells following CSE exposure (Figure 4A). In addition, the expression levels of cleaved caspase-3 and -8 proteins were increased in CSE-induced cells, while treatment with roflumilast markedly decreased the expression of these proteins (Figure 4C). Taken together, these results suggest that roflumilast confers protection against CSE-induced apoptotic cell death in Beas-2B cells. Previous studies have reported a functional role for autophagic proteins in CS-induced epithelial cell death24,25. Cultured epithelial cells exposed to CSE responded with increased autophagosome formation and the accumulation of LC3B-II. CSE induced the extrinsic apoptosis pathway in epithelial cells and the downstream activation of pro-apoptotic caspases. A genetic deletion study of crucial autophagy proteins (i.e., Beclin 1 and LC3B) revealed an inhibition in apoptosis in response to CSE exposure in vitro, suggesting that increased autophagy, to a certain extent, occurred in association with epithelial cell death and also may have cross talk between them24,25. Therefore, an emerging hypothesis is that mitophagy may also contribute to cell death in a context-specific fashion. Although published studies have indicated a relationship between necroptosis and the disintegration of mitochondria (mitophagy), the precise mechanism remains to be elucidated38. Several reports have implicated altered mitochondria as downstream effectors of necrosome activation39,40. In this study, we only investigated the role of roflumilast on mitophagy-dependent cell death, although DRP1-dependent mitophagy in response to CSE can also act as an upstream regulator of apoptotic cell death. DRP1- or PINK1-knockdown cells displayed a significant reduction in cell death in response to CSE exposure relative to control siRNA-transfected cells.
In conclusion, in bronchial epithelial cells, roflumilast diminished CSE-induced cell death by regulating mitophagy-related proteins. Similar to Mdivi-1 (a known inhibitor of mitochondrial fission), roflumilast may confer cytoprotection against CSE by inhibiting mitochondrial fission. These results suggest the potential utility of roflumilast as a preventive therapeutic targeting cell death in addition to its anti-inflammatory action in the treatment of emphysema and COPD.
Acknowledgments
This work was supported by Research Grants (FRD2014-10-2) funded by Gachon University Gil Medical Center in 2015 to Jeong-Woong Park.
References
1. Dal-Re R. Worldwide behavioral research on major global causes of mortality. Health Educ Behav 2011;38:433-440. PMID: 21558465.


2. Barnes N, Calverley PM, Kaplan A, Rabe KF. Chronic obstructive pulmonary disease and exacerbations: clinician insights from the global Hidden Depths of COPD survey. Curr Med Res Opin 2014;30:667-684. PMID: 24256026.


3. Barnes PJ, Shapiro SD, Pauwels RA. Chronic obstructive pulmonary disease: molecular and cellular mechanisms. Eur Respir J 2003;22:672-688. PMID: 14582923.


4. Park JW, Ryter SW, Choi AM. Functional significance of apoptosis in chronic obstructive pulmonary disease. COPD 2007;4:347-353. PMID: 18027162.


5. Slebos DJ, Ryter SW, van der, Liu F, Guo F, Baty CJ, et al. Mitochondrial localization and function of heme oxygenase-1 in cigarette smoke-induced cell death. Am J Respir Cell Mol Biol 2007;36:409-417. PMID: 17079780.


6. Park JW, Kim HP, Lee SJ, Wang X, Wang Y, Ifedigbo E, et al. Protein kinase C alpha and zeta differentially regulate death-inducing signaling complex formation in cigarette smoke extract-induced apoptosis. J Immunol 2008;180:4668-4678. PMID: 18354190.


7. Kim HP, Wang X, Chen ZH, Lee SJ, Huang MH, Wang Y, et al. Autophagic proteins regulate cigarette smoke-induced apoptosis: protective role of heme oxygenase-1. Autophagy 2008;4:887-895. PMID: 18769149.


8. Youle RJ, Narendra DP. Mechanisms of mitophagy. Nat Rev Mol Cell Biol 2011;12:9-14. PMID: 21179058.




9. Mizumura K, Cloonan SM, Nakahira K, Bhashyam AR, Cervo M, Kitada T, et al. Mitophagy-dependent necroptosis contributes to the pathogenesis of COPD. J Clin Invest 2014;124:3987-4003. PMID: 25083992.



10. Sureshbabu A, Bhandari V. Targeting mitochondrial dysfunction in lung diseases: emphasis on mitophagy. Front Physiol 2013;4:384PMID: 24421769.



11. Frank M, Duvezin-Caubet S, Koob S, Occhipinti A, Jagasia R, Petcherski A, et al. Mitophagy is triggered by mild oxidative stress in a mitochondrial fission dependent manner. Biochim Biophys Acta 2012;1823:2297-2310. PMID: 22917578.


12. Boswell-Smith V, Spina D. PDE4 inhibitors as potential therapeutic agents in the treatment of COPD-focus on roflumilast. Int J Chron Obstruct Pulmon Dis 2007;2:121-129. PMID: 18044684.


13. Torphy TJ. Phosphodiesterase isozymes: molecular targets for novel antiasthma agents. Am J Respir Crit Care Med 1998;157:351-370. PMID: 9476844.


14. Calverley PM, Rabe KF, Goehring UM, Kristiansen S, Fabbri LM, Martinez FJ, et al. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet 2009;374:685-694. PMID: 19716960.


15. Cortijo J, Iranzo A, Milara X, Mata M, Cerda-Nicolas M, Ruiz-Sauri A, et al. Roflumilast, a phosphodiesterase 4 inhibitor, alleviates bleomycin-induced lung injury. Br J Pharmacol 2009;156:534-544. PMID: 19154443.



16. Lee JS, Park SJ, Cho YS, Huh JW, Oh YM, Lee SD. Role of AMP-activated protein kinase (AMPK) in smoking-induced lung inflammation and emphysema. Tuberc Respir Dis 2015;78:8-17.

17. Uh ST, Koo SM, Kim YK, Kim KU, Park SW, Jang AS, et al. Inhibition of vitamin d receptor translocation by cigarette smoking extracts. Tuberc Respir Dis 2012;73:258-265.

18. Le Quement C, Guenon I, Gillon JY, Valenca S, Cayron-Elizondo V, Lagente V, et al. The selective MMP-12 inhibitor, AS111793 reduces airway inflammation in mice exposed to cigarette smoke. Br J Pharmacol 2008;154:1206-1215. PMID: 18493250.



19. Martorana PA, Lunghi B, Lucattelli M, De Cunto G, Beume R, Lungarella G. Effect of roflumilast on inflammatory cells in the lungs of cigarette smoke-exposed mice. BMC Pulm Med 2008;8:17PMID: 18755021.




20. Kwak HJ, Park KM, Choi HE, Chung KS, Lim HJ, Park HY. PDE4 inhibitor, roflumilast protects cardiomyocytes against NO-induced apoptosis via activation of PKA and Epac dual pathways. Cell Signal 2008;20:803-814. PMID: 18276108.


21. Park JW, Ryter SW, Kyung SY, Lee SP, Jeong SH. The phosphodiesterase 4 inhibitor rolipram protects against cigarette smoke extract-induced apoptosis in human lung fibroblasts. Eur J Pharmacol 2013;706:76-83. PMID: 23499692.


22. Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell 2011;147:728-741. PMID: 22078875.


23. Zhang J. Autophagy and mitophagy in cellular damage control. Redox Biol 2013;1:19-23. PMID: 23946931.



24. Chen ZH, Lam HC, Jin Y, Kim HP, Cao J, Lee SJ, et al. Autophagy protein microtubule-associated protein 1 light chain-3B (LC3B) activates extrinsic apoptosis during cigarette smoke-induced emphysema. Proc Natl Acad Sci U S A 2010;107:18880-18885. PMID: 20956295.



25. Chen ZH, Kim HP, Sciurba FC, Lee SJ, Feghali-Bostwick C, Stolz DB, et al. Egr-1 regulates autophagy in cigarette smokeinduced chronic obstructive pulmonary disease. PLoS One 2008;3:e3316. PMID: 18830406.



26. Nakahira K, Cloonan SM, Mizumura K, Choi AM, Ryter SW. Autophagy: a crucial moderator of redox balance, inflammation, and apoptosis in lung disease. Antioxid Redox Signal 2014;20:474-494. PMID: 23879400.



27. Springer W, Kahle PJ. Regulation of PINK1-Parkin-mediated mitophagy. Autophagy 2011;7:266-278. PMID: 21187721.


28. Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J 2008;27:433-446. PMID: 18200046.



29. Lee Y, Lee HY, Hanna RA, Gustafsson AB. Mitochondrial autophagy by Bnip3 involves Drp1-mediated mitochondrial fission and recruitment of Parkin in cardiac myocytes. Am J Physiol Heart Circ Physiol 2011;301:H1924-H1931. PMID: 21890690.



30. Givvimani S, Munjal C, Tyagi N, Sen U, Metreveli N, Tyagi SC. Mitochondrial division/mitophagy inhibitor (Mdivi) ameliorates pressure overload induced heart failure. PLoS One 2012;7:e32388. PMID: 22479323.



31. Zhang X, Yan H, Yuan Y, Gao J, Shen Z, Cheng Y, et al. Cerebral ischemia-reperfusion-induced autophagy protects against neuronal injury by mitochondrial clearance. Autophagy 2013;9:1321-1333. PMID: 23800795.


32. Vazquez-Martin A, Cufi S, Corominas-Faja B, Oliveras-Ferraros C, Vellon L, Menendez JA. Mitochondrial fusion by pharmacological manipulation impedes somatic cell reprogramming to pluripotency: new insight into the role of mitophagy in cell stemness. Aging (Albany NY) 2012;4:393-401. PMID: 22713507.



33. Gharanei M, Hussain A, Janneh O, Maddock H. Attenuation of doxorubicin-induced cardiotoxicity by mdivi-1: a mitochondrial division/mitophagy inhibitor. PLoS One 2013;8:e77713. PMID: 24147064.



34. Demedts IK, Demoor T, Bracke KR, Joos GF, Brusselle GG. Role of apoptosis in the pathogenesis of COPD and pulmonary emphysema. Respir Res 2006;7:53PMID: 16571143.




35. Imai K, Mercer BA, Schulman LL, Sonett JR, D'Armiento JM. Correlation of lung surface area to apoptosis and proliferation in human emphysema. Eur Respir J 2005;25:250-258. PMID: 15684288.


36. Rangasamy T, Misra V, Zhen L, Tankersley CG, Tuder RM, Biswal S. Cigarette smoke-induced emphysema in A/J mice is associated with pulmonary oxidative stress, apoptosis of lung cells, and global alterations in gene expression. Am J Physiol Lung Cell Mol Physiol 2009;296:L888-L900. PMID: 19286929.



37. Kosmider B, Messier EM, Chu HW, Mason RJ. Human alveolar epithelial cell injury induced by cigarette smoke. PLoS One 2011;6:e26059PMID: 22163265.



38. Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol 2010;11:700-714. PMID: 20823910.



39. Wang Z, Jiang H, Chen S, Du F, Wang X. The mitochondrial phosphatase PGAM5 functions at the convergence point of multiple necrotic death pathways. Cell 2012;148:228-243. PMID: 22265414.


40. Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC, et al. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science 2009;325:332-336. PMID: 19498109.


Figure 1
Effects of cigarette smoke extract (CSE) on mitochondrial dysfunction and mitophagy in Beas-2B epithelial cells. Human bronchial epithelial (Beas-2B) cells were treated with the indicated percent of CSE for 24 hours. (A) Flow cytometric analysis of CSE-treated Beas-2B cells left unstained or labeled with tetramethylrhodamine, ethyl ester (TMRE) and MitoSOX Red. Cells were induced with 10% CSE for 18 hours. (B, C) The expression of mitophagy-related proteins was determined by Western blot analysis. DRP1: dynamin-1-like protein; PINK1: PTEN-induced putative kinase-1.

Figure 2
Effects of dynamin-1-like protein (DRP1) and PTEN-induced putative kinase-1 (PINK1) silencing on cigarette smoke extract (CSE)-induced cell death in Beas-2B epithelial cells. Beas-2B cells were exposed to different concentrations of CSE for 18 hours. Cell viability was evaluated using the MTT assay and cytotoxicity was measured by the lactate dehydrogenase (LDH) release test. Beas-2B cells were pre-treated with control, DRP1, or PINK1 siRNAs for 48 hours prior to treatment with 10% CSE for 18 hours. Cell cytotoxicity (C) and viability (D) were estimated by the LDH and MTT assays, respectively. The data shown represent the mean±SD derived from three determinations. *p<0.05 and ***p<0.001, compared with the control siRNA-transfected group.
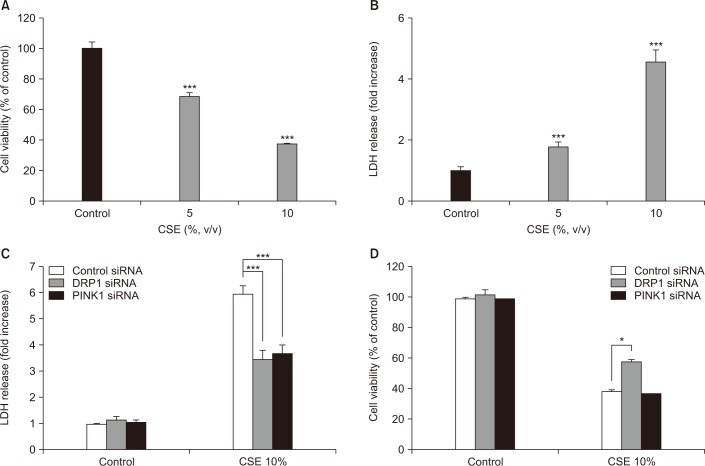
Figure 3
Effects of roflumilast on mitochondrial dysfunction and mitophagy in cigarette smoke extract (CSE)-induced Beas-2B epithelial cells. Beas-2B cells were pre-treated for 2 hours with 5 µM roflumilast and exposed to 10% CSE for 4 hours. (A) Detection of mitochondrial dysfunction (×400). Cells labeled with tetramethylrhodamine, ethyl ester (TMRE) or MitoSOX Red were incubated with control or 5 µM roflumilast and treated with 10% CSE for 4 hours. (B) The expression of mitophagy-related proteins was determined by Western blot analysis. (C) The expression of apoptosis-related proteins was determined by Western blot analysis. DRP1: dynamin-1-like protein; PINK1: PTEN-induced putative kinase-1; R5: roflumilast 5 µM.

Figure 4
Effects of roflumilast on apoptotic cell death in cigarette smoke extract (CSE)-induced Beas-2B epithelial cells. Cells were pre-treated for 2 hours with 5 µM roflumilast followed by treatment with 10% CSE for 18 hours. Cell viability (A) and cytotoxicity (B) were estimated by MTT and lactate dehydrogenase (LDH) assay, respectively. (C) Beas-2B cells were pre-treated with control, dynamin-1-like protein (DRP1), or PTEN-induced putative kinase-1 (PINK1) siRNAs for 48 hours prior to treatment with 10% CSE for 18 hours. R5: roflumilast 5 µM. The data shown represent the mean±SD derived from three determinations. *p<0.05, **p<0.01, and ***p<0.001, compared with the CSE-treated group.



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Print
Print Download Citation
Download Citation




