The Prognostic Value of the Charlson's Comorbidity Index in Patients with Prolonged Acute Mechanical Ventilation: A Single Center Experience
Article information
Abstract
Background
The aim of our study was to evaluate the prognostic value of Charlson's weighted index of comorbidities (WIC) in patients with prolonged acute mechanical ventilation (PAMV, ventilator care ≥96 hours).
Methods
We retrospectively enrolled 299 Korean PAMV patients who were admitted in a medical intensive care unit (ICU) of a university-affiliated tertiary care hospital between 2008 and 2013. Survivors were defined as patients who survived for 60 days after ICU admission.
Results
The patients' mean age was 65.1±14.1 years and 70.6% were male. The mean ICU and hospital length of stay was 21.9±19.7 and 39.4±39.1 days, respectively. In addition, the 60-day mortality rate after ICU admission was 35.5%. The mean WIC was 2.3±1.8, with significant differences between nonsurvivors and survivors (2.7±2.1 vs. 2.1±1.7, p<0.05). The area under the curve of receiver-operating-characteristics curve for WIC was 0.593 (95% confidence interval [CI], 0.523–0.661; p<0.05). Based on Kaplan-Meier curves of 60-day survival, WIC ≥5 had statistically lower survival than WIC <5 (log-rank test, p<0.05). In a multivariate Cox proportional hazard model, WIC ≥5 was associated with poor prognosis (hazard ratio, 1.901; 95% CI, 1.140–3.171; p<0.05). The mortality rate of patients with WIC ≥5 was 54.2%.
Conclusion
Our study showed a WIC score ≥5 might be helpful in predicting 60-day mortality in PAMV patients.
Introduction
Among the patients admitted to intensive care units (ICUs), those requiring ventilator care consume high amounts of medical resources and incur much expense12. Several studies have suggested that an important factor contributing to the high cost of health care may be associated with the clinician decision-making at the patient's bedside3456. Accordingly, the length of stay of a patient in the ICU would be expected to be longer, and it may be helpful for critical-care physicians to determine at an early stage if these patients would really benefit from ICU care.
Recently, some reports described a novel subpopulation of patients on mechanical ventilation (MV): those who require MV for ≥96 hour, referred to as "prolonged acute mechanical ventilation" (PAMV) cases2789. These studies reported that PAMV patients constituted one-third of all hospitalized patients undergoing MV and consumed two thirds of the medical resources of all MV patients2789. Also, these patients are projected to increase in the years to come78. Thus, further research needs to focus on more accurately characterizing the clinical outcomes and prognostic factors for such patients.
When critical care physicians discuss and guide the goal of care and prognosis of PAMV patients, a mortality prediction model would be necessary to address prognostic uncertainty. Although clinical parameters on their clinical course would be associated with clinical outcomes, the concurrent comorbidities before admission should be considered. In the existing literature, Charlson's weighted index of comorbidities (WIC) has been suggested to have a predictive value10 and has also been shown to predict hospital mortality in critically ill patients1011121314. In existing literature, there were some reports about the validity and utility of Charlson's WIC in relation to other illness scores (such as Acute Physiology and Chronic Health Evaluation [APACHE] II and Sequential Organ Failure Assessment [SOFA] scores) in predicting the outcome of critically ill patients111213141516. Based on previous reports, we hypothesized the role of this index as a prognostic model for PAMV population.
This study sought to investigate the prognostic value of Charlson's WIC in predicting 60-day mortality after MV in patients defined as PAMV cases in a medical ICU of a university-affiliated tertiary care center in South Korea.
Materials and Methods
1. Study design
This retrospective study was conducted in Pusan National University Hospital, Republic of Korea, a university-affiliated tertiary care hospital with a 12-bed medical ICU with full cardiovascular and close airway monitoring as well as a separate seven-bed adult coronary care unit. The physician staff in the medical ICU consisted of one full-time specialist, one clinical fellow in pulmonary and critical care medicine, two resident physicians, and one intern. Fellows and resident physicians provided overnight care, and the nurse-to-bed ratio was 1:3. Full-time physical and respiratory rehabilitation therapies were available for all patients. Consultation services were accessible for all the subspecialists in the hospital. All patients were managed according to therapeutic recommendations based on early goal-directed therapy and a lung-protective ventilator strategy1718.
2. Study subjects
Patients defined as PAMV cases within the period from March 1, 2008, to February 28, 2013 were included in this study. The PAMV patients were those who had undergone MV for longer than 4 days in the ICU2, regardless of tracheostomy. The exclusion criteria included patients younger than 18 years who received ventilator care prior to ICU admission. The medical records and laboratory and radiological findings of all the patients were reviewed. The investigators completed a case report form, and data were collected from March to June 2014. The three investigators (S.E.S., S.H.L., and K.L.) confirmed that the study objectives and procedures were honestly disclosed, and all had full access to all the study data. This study was conducted with approval from the Institutional Review Board of Pusan National University Hospital (E-2015127). This study had no impact on the patient treatment.
3. Data collection
The following data were gathered from the medical records of each patient: age, gender, body mass index, presence of shock on the ICU admission day, duration of MV, and length of stay in the ICU and hospital. The ICU and in-hospital mortality for all the patients were recorded. The APACHE II and SOFA scores were calculated to quantify the severity of the illness and the degree of organ dysfunction, respectively, using the data from the first 24 hours of ICU admission1516. Charlson's WIC, acquired from the medical records of all the patients' comorbidities before the catastrophic illness requiring ICU care, was calculated to predict the effect of the underlying diseases on the patient outcomes10. The major diagnoses leading to MV were also collected. We also evaluated the do-not-resuscitate (DNR) status records of the patients within 4 days after ICU admission. The survivors were defined as patients who had survived for 60 days after ICU admission.
4. Statistical analysis
The continuous variables were expressed as median values with the range or mean±standard deviation, and the categorical variables were expressed as numbers (percentages). Student's t test and Mann-Whitney U test were used to compare the continuous variables, and the chi-square and Fisher exact tests (for small numbers) were used to compare the categorical variables. To evaluate the relationship between WIC and the other clinical variables, the Pearson's correlation coefficient (γ) was calculated. Receiver operating characteristic (ROC) curves and the area under the curve (AUC) were used. Univariate Cox proportional hazard models were developed to determine the relationship between WIC and mortality. Variables with p<0.05 were included in the multivariate Cox proportional hazard model. Survival curves were prepared using the Kaplan-Meier method with the log-rank test. All the statistical analyses were performed using the Statistical Package for the Social Sciences (version 19.0, IBM Corp., Armonk, NY, USA) program. A two-tailed p-value <0.05 was considered to indicate a significant difference.
Results
A total of 754 patients who had been admitted to the medical ICU and to whom MV was applied during the study period were identified. Among them, 311 patients (41.2%) met the definition of PAMV. Among these patients, 12 patients (3.9%) had DNR order given before day 4 of MV. We appropriately excluded them because this would be effective modified of prognosis. For the total enrolled patients (n=299), the ICU and in-hospital mortality rates were 41.5 and 42.1%, respectively. In addition, the 60-day mortality rate after ICU admission was 35.5%. Table 1 shows the results of the baseline characteristics of all enrolled patients, and the results of the comparisons between the survivors and the nonsurvivors.
The enrolled patients had an average WIC of 2.3±1.8 and a median WIC of 2 (range, 0–11). The number of patients for each WIC level and the corresponding mortality are shown in Figure 1. Results of the investigation of all the components of WIC and their matching patients are shown in Table 2. Table 2 also shows the comparisons between survivors and nonsurvivors according to the components of WIC.

The number of patients for each Charlson's weighted index of comorbidities (WIC) score (left Y axis) and the corresponding mortality (right Y axis).

Comparison of the components of Charlson's weighted index of comorbidities between survivors and nonsurvivors
When the relationship between WIC and the clinical variables upon admission were analyzed, it was found that the WIC score was positively correlated with the APACHE II (γ=0.321, p<0.001) and SOFA (γ=0.224, p<0.001) scores on admission day, respectively.
When the WIC scores of the nonsurvivors and survivors were compared, it was found that the nonsurvivors had significantly higher WIC scores than the survivors (2.7±2.1 vs. 2.1±1.7, p=0.008). The AUC of the ROC curve for WIC in our cohort was 0.592 (95% confidence interval, 0.523–0.661; p=0.008). Kaplan-Meier curves of 60-day survival are shown in Figure 2. Survival curves showed WIC ≥5 had statistically significant differences than other WIC scores (log-rank test, p=0.027). In a univariate Cox proportional hazard model, four clinical factors were associated with poor prognosis (p<0.05): WIC more than or equal to 5; presence of shock on ICU admission day; acute respiratory distress syndrome (ARDS) on ICU admission day; and APACHE II score of more than or equal to 17 (the cut-off level was determined by ROC curves).
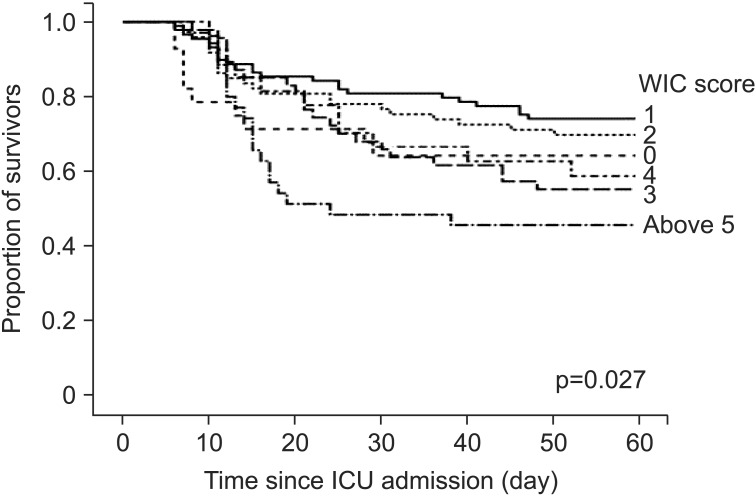
Kaplan-Meier survival curves of 60-day mortality according to the number of Charlson' weighted index of comorbidities (WIC). ICU: intensive care unit.
After adjusting for age and sex, these factors were included in a multivariate model: 3 factors (WIC ≥5, ARDS on ICU admission day, and APACHE II ≥17) were independently associated with the 60-day mortality (Table 3). The mortality rate of patients with WIC ≥5 was 54.2%.
Discussion
The previous studies have shown that comorbid conditions before admission appear to predict mortality in ICU patients, and as such, comorbidity indices should be used as an important tool for predicting patient outcomes11192021. In the present study, the prognostic value of Charlson's WIC in PAMV patients was investigated. It was demonstrated that a WIC score of more than or equal 5 was associated with the increased 60-day mortality of our enrolled patients. Based on the present study results, a higher WIC score might be helpful in predicting the mortality of PAMV patients.
In this study, however, the AUC of the ROC curve for the WIC score was lower and the WIC score had weak correlation with the APACHE II and SOFA score. Moreover, as shown in Figure 2, we could not find the prognostic value in a WIC score less than 4 in predicting 60-day mortality. Also, we could not find how each component of WIC could affect their prognosis. Although our result suggests that WIC may be a useful indicator to assist physicians in decisions related to patient management and counseling with their families or surrogates, this index should not override clinical judgments. In addition, our study was a single center experience. Further research is needed to determine the influence of comorbidities on the prognosis of these patients.
Based on the previous reports2789, the incidence of PAMV cases seems likely to increase in the future, making it an emerging challenge for the critical-care delivery system. In the Asian ICUs (including those in South Korea), however, the critical-care resources and facilities of university hospitals are typically limited compared to those of the developed countries in North America and Europe2223. In addition, a shortage of critical-care personnel, including full-time intensivists, and a low nurse-to-patient ratio, exist, which are serious issues for clinical practice24. Accordingly, a prediction model that identifies patients with poor or good risks for clinical course may be useful for informing the discussions among the clinicians and the patients' families (or surrogates). Although there were reported data about the prognostic factors for these patients25, developing a new mortality model through a large-scale multicenter study would be necessary for the South Korean patient population requiring PAMV.
This study had several limitations. First, it was conducted retrospectively, which may have resulted in an informational bias. Second, the study was an observational retrospective analysis, and our data represent the experience of a single center. Thus, the results of our study may not represent the general situation in Korea. Third, our study was a retrospective one, we could not know how to deal with some patients who were not already indicated in the ICU but could not be out of the ICU because there was no general ward vacancy or availability in other hospitals. Fourth, in our results, the mortality of patients with WIC=0 was higher than that of patients with WIC=1 or 2. Because our study was retrospective study, all patients' various comorbidities could be omitted in medical records, and all comorbidities could not gathered completely.
In conclusion, this study investigated the usefulness of Charlson's WIC as a prognostic indicator in PAMV patients. It was found that higher WIC scores than that of the survivors, and WIC ≥5 might be helpful in predicting 60-day mortality. In terms of identifying a prognostic value of this score in PAMV, this population is set to increase so prospective, large-scale, multicenter studies with longer follow-up time periods are required to determine if this index can be considered a prognostic factor.
Notes
Conflicts of Interest: No potential conflict of interest relevant to this article was reported.




