Influence of Environmental Exposures on Patients with Chronic Obstructive Pulmonary Disease in Korea
Article information
Abstract
Background
Chronic obstructive pulmonary disease (COPD) is characterized by airflow limitation and results from environmental factors and genetic factors. Although cigarette smoking is a major risk factor, other environmental exposures can influence COPD. The purpose of this study is to investigate the clinical characteristics of COPD according to the history of environmental exposure.
Methods
The study population comprised of 347 subjects with COPD who were recruited from the pulmonary clinics of 14 hospitals within the Korean Obstructive Lung Disease Study Group. We classified environmental exposures according to history of living near factory, and direct exposure history to firewood or briquette. According to living environmental exposures, we compared the frequency of respiratory symptoms, pulmonary function, quality of life, exercise capacity, and computed tomography phenotypes.
Results
Thirty-one subjects (8.9%) had history of living near factory, 271 (78.3%) had exposure history to briquette, and 184 (53.3%) had exposure history to firewood. Patients with history of living near a factory had a significantly longer duration of sputum, while patients with exposure to firewood tended to have lower forced expiratory volume in one second, and patients with exposure to briquette tended to have lower six minute walk distance.
Conclusion
COPD subjects with the history of living near factory had more frequent respiratory symptoms such as sputum. Our data suggest that environmental exposure may influence clinical phenotype of COPD.
Introduction
Chronic obstructive lung disease (COPD) is a leading cause of morbidity and mortality worldwide1. It is a chronic inflammatory disease characterized by persistent airflow limitation, and developed from chronic exposures of environmental factors in genetically susceptible individuals2. Although cigarette smoking is known a major risk factor, recent evidence indicates that other environmental exposures, such as air pollutants and workplace exposures, can influence COPD3.
COPD shows heterogeneous features that may present distinct clinical presentation and disease progression. Therefore, environmental exposures besides cigarette smoking may induce specific respiratory conditions or atypical characteristics of COPD. A patient series of COPD showed that occupational inhalant exposure was associated with specific features of COPD4. However, regarding for clinical characteristics of patients with COPD, it is generally known only about influence of cigarette smoking.
It is little known about distinct clinical characteristics of patients with COPD according for other countries. There are only a few reports about population based prevalence or risk factors in COPD. It is warrant to study about clinical characteristics and environmental influences according for other countries or regions. To date, it is little known about clinical features by environmental exposures in Korean patients with COPD.
The purpose of this study is to investigate clinical characteristics of patients with COPD according to history of environmental exposures.
Materials and Methods
1. Study population
The data of 347 patients diagnosed with COPD were analyzed retrospectively. These patients were selected from the Korean Obstructive Lung Disease (KOLD) Cohort, which consisted of 439 stable patients with obstructive lung disease who had been prospectively recruited from the pulmonary clinics of 16 hospitals in South Korea between June 2005 and July 2012. The inclusion criteria for KOLD patients have been described previously5. The patients were diagnosed with COPD if they were aged >45 years, had >10 pack-years of cigarette smoking, and had a post-bronchodilator forced expiratory volume in one second (FEV1)/forced vital capacity<0.7, but did not have bronchiectasis or sequelae of pulmonary tuberculosis.
At the enrollment visit, all patients were evaluated with medical interviews, physical examinations, spirometry, bronchodilator reversibility tests, and lung volume, and six-minute walk tests. Health-related quality of life was evaluated by calculating the total score of St. George's Respiratory Questionnaire (SGRQ). Dyspnea was evaluated using the modified Medical Research Council Dyspnea grade. Cough was evaluated using the question, "Do you usually have cough, for more than three days during a week?" and sputum was evaluated with the question, "Do you usually have sputum, for more than three days during a week?" Wheeze was evaluated with the question, "Have you ever had wheezing or whistling in your chest?"
In addition, volumetric computed tomography (CT) was performed to evaluate airway wall thickness, emphysema severity, and mean lung density (MLD) ratio at full expiration and inspiration.
This study was approved by the Institutional Review Board of Asan Medical Center (approval No. 2005-0345) and of other 15 hospitals. Individual informed written consent was obtained from all patients.
2. Pulmonary function tests
The method for pulmonary function tests have been described previously5. Spirometry was performed by using a Vmax 22 (Sensor-Medics, Yorba Linda, CA, USA) or a PFDX (MedGraphics, St Paul, MN, USA). To assess post-bronchodilator FEV1 increases, spirometry was performed before bronchodilation and 15 minutes after inhalation of salbutamol 400 µg through a metered-dose inhaler with a spacer. Bronchodilator reversibility was evaluated by measuring post-bronchodilator FEV1 increase in liters. Lung volumes were measured by body plethysmography (V6200; Sensor-Medics or PFDX). Diffusing capacity for carbon monoxide (DLco) was measured by the single-breath method using a Vmax229D (Sensor-Medics) or a Masterlab Body (JaegerAB, Würtsburg, Germany). All pulmonary function tests were performed as recommended by the American Thoracic Society (ATS)/European Respiratory Society (ERS).
3. Computed tomography
Volumetric CT scans were obtained by using a 16-multidetector CT scanner (Somatom Sensation instrument; Siemens, Erlangen, Germany; GE Lightspeed Ultra instrument; General Electric Healthcare, Milwaukee, WI, USA; Philips Brilliance instrument; Philips Medical Systems, Best, The Netherlands) as previously described6. The volume fraction (%) of the lung below -950 hounsfield units (HU) at full inspiration was calculated automatically (inspiratory V950) from the CT data. The ratio of MLD on expiration and inspiration was calculated. The airway dimensions, wall area (WA), lumen area (LA), and wall area percent [WA%; i.e., WA/(WA+LA)×100] were measured near the origin of two segmental bronchi (the right apical and left apico-posterior) that were selected by consensus by two radiologists.
4. Measurement for environmental exposures
The environmental exposures were divided as follows: a history of living near factory, direct exposure history to firewood, and direct exposure history to briquette. A history of living near factory was evaluated using the question, "Have you ever been live near factory for a lifetime, or now you are live near factory?" The question for direct exposure history to firewood was "For cooking and/or heating, have you ever been exposed to fuels of wood for one year or more?" and the question for direct exposure history to briquette was "For cooking and/or heating, have you ever been exposed to fuels of charcoal for one year or more?"
According to environmental exposures, we compared the frequency of respiratory symptoms, pulmonary function, quality of life questionnaire, exercise capacity, and CT phenotypes.
5. Statistical analysis
The descriptive statistics of the clinical variables are expressed as means and standard deviations, and the Student's t- and chi-square tests were used to confirm statistical significance between the environmental exposures. And we evaluated these data with adjusting gender, age, body mass index (BMI), and smoking, using the statistical software SAS version 9.2 (SAS Inc., Cary, NC, USA). p-values of less than 0.05 were considered significant.
Results
1. Demographic characteristics
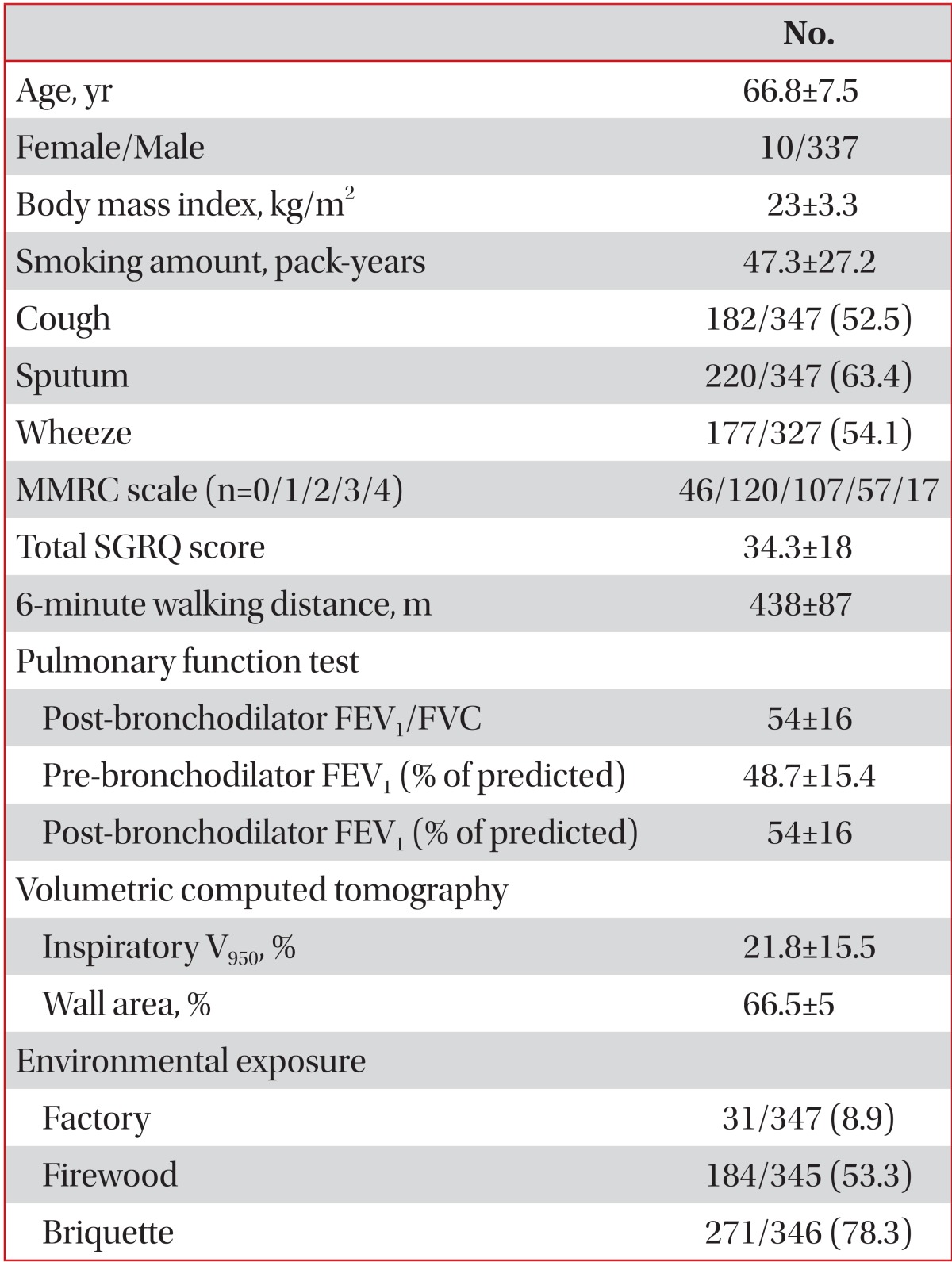
The demographic characteristics of 347 patients (337 males, 10 females) were represented. (Table 1). The mean age of patients with COPD was 66.8 years (standard deviation, 7.5), the mean BMI was 23.0 (3.3), and the mean smoking amount was 47.3 (27.2) pack-years. Thirty-one patients (8.9%) had history of living near factory, 184 patients (53.3%) had exposure history to firewood, and 271 patients (78.3%) had exposure history to briquette.
2. Difference of clinical characteristics according to environmental exposures
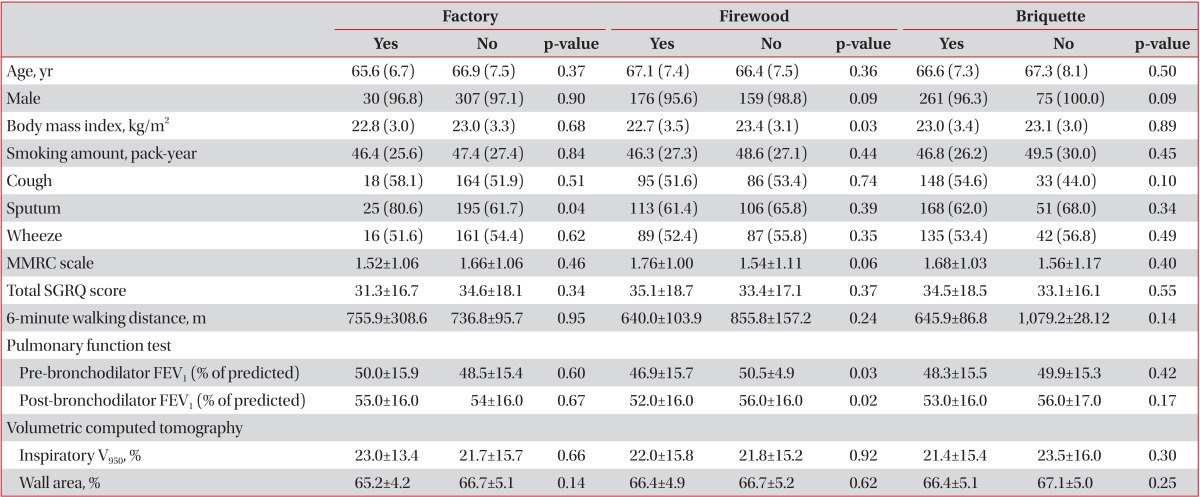
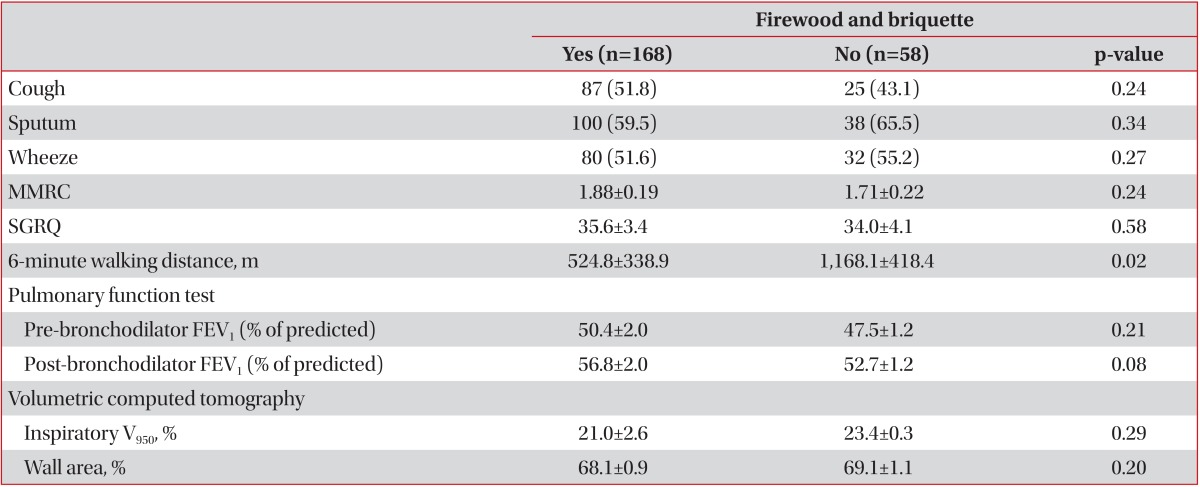
On univariate analysis, patients with history of living near factory had significantly longer duration of sputum and patients with exposure to firewood had lower BMI and lower FEV1, but patients with exposure to briquette showed no difference (Table 2). Patient with co-exposure to firewood and briquette had lower FEV1 (Table 3).
Univariate analysis for difference of clinical characteristics by co-exposure to firewood and briquette
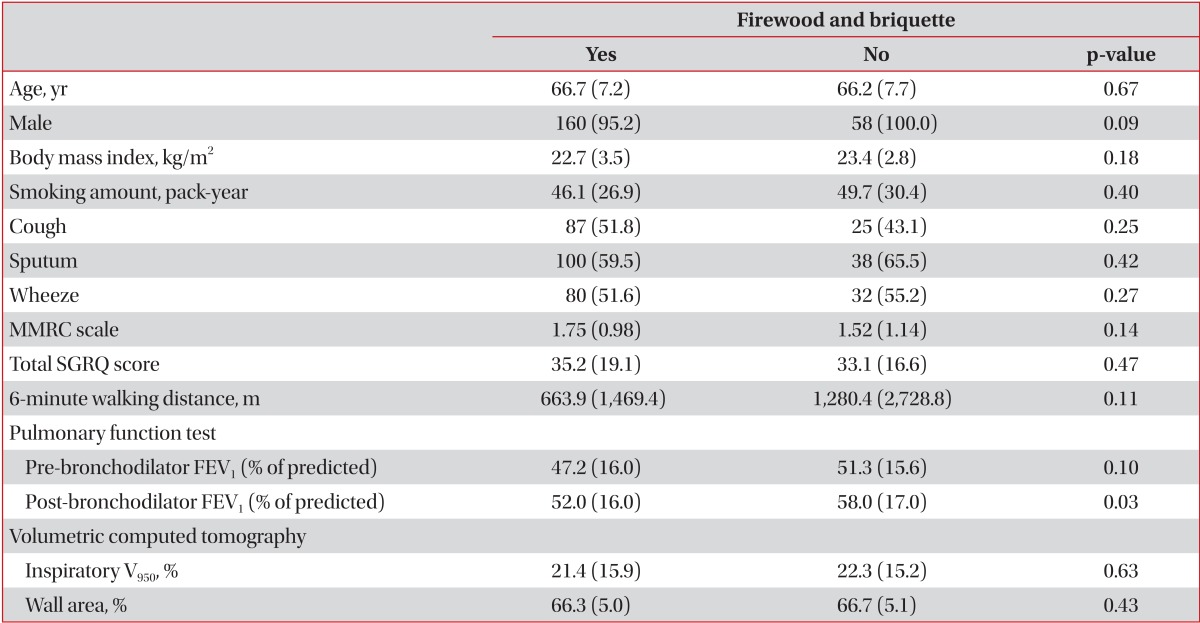
On adjusting age, gender, BMI, and smoking, patients with history of living near factory also showed longer duration of sputum (Table 4). Patients with exposure to firewood tended to have lower FEV1 (p=0.09), briquette tended to have lower six minute walk distance (p=0.06). And patients with co-exposure to firewood and briquette had lower 6 minute walking distance (Table 5).
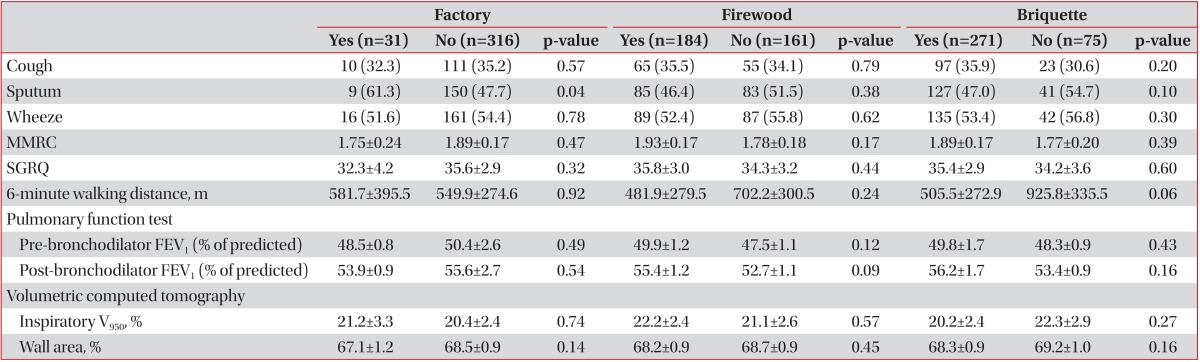
Difference of clinical characteristics according to environmental exposures, adjusting gender, age, BMI, and smoking
Discussion
In the current study, we show that the history of living near factory was associated with more frequent respiratory symptoms such as sputum and the exposure to firewood or briquette tend to be associated with poor lung function in a Korean COPD cohort group. Our data suggest that various environmental exposures may influence clinical features of COPD.
Although COPD is characterized by airflow limitation, it is widely recognized that significant heterogeneity exists with respect to clinical presentation, imaging, and response to therapy7. It is thought that clinical phenotypes of COPD are developed from various exposures to environmental factors and various responses of individuals having different genetic susceptibility. Nevertheless, it is the reality that most clinical trials in COPD recruit only cigarette smokers since cigarette smoking is known as a major risk factor8. Therefore, the majority of knowledge about clinical characteristics of patients with COPD is based on its influence of cigarette smoking. However, emerging evidence has suggested that other risk factors, such as air pollutants and workplace exposure, are strongly associated with COPD3. To date, it is little known how environmental exposures interact with clinical characteristics of patients with COPD.
A few results on the association between environmental exposures and specific features of COPD have been reported. In never smoking patients, dust exposure on occupation was not associated with chronic bronchitis in an European survey, but was significantly associated with spirometry-defined COPD in United States9,10. And, young COPD patients with occupational exposure showed increased work-related respiratory disability, more asthma-like symptoms, and atopy, regardless of smoking4. Recently, an Asian COPD cohort reported that the characteristics of COPD patients varied and history of exposure to biomass fuels or dusty jobs was related to the frequency of symptoms, severe airflow limitation, and poor quality of life11. Our study also shows that history of living near factory was associated with more frequent respiratory symptoms while firewood exposure was poor lung function.
Recently, several cases of constrictive bronchiolitis, a rare respiratory condition, were reported among soldiers with a history of inhalational exposure to sulfur-mine fires12. This suggests that exposure to a particular inhalational dust may induce a specific respiratory condition and symptoms. However, to date, there is insufficient evidence to support an association between specific environmental exposure and distinct clinical outcomes of COPD. It seems to be very difficult for establishing causal relationship between environmental exposure and clinical features of COPD, because environmental exposures on patients are almost impossible to measure quantitatively, as its chronic and multi-factorial nature. Collection of cohort studies regarding environmental exposures, like our study, may lead us close to answer for causal relationship between environmental exposure and clinical features of COPD.
This study has several limitations. First, the intensity and duration for past exposure to environmental exposure may not be accurate quantitatively because it is collected only based on participant memory and questionnaires. A long term follow up study design and using exposure metrics is warranted. Second, the statistical power for the analysis may be low because numbers of subjects with environmental exposure were small for showing significant difference, especially the history of living near factory. More large scale or population based study will help for identifying effects of environmental exposures on patients with COPD. Third, there were no matching control groups. Control groups of different regions or groups with no exposure for firewood or briquette could be compared. It could make clear more about correlation with environmental exposure and clinical features on COPD patients.
In conclusion, COPD subjects with the history of living near factory had more frequent respiratory symptoms such as sputum. Our data suggest that environmental exposure may influence clinical phenotype of COPD.
Acknowledgements
This study was supported by a grant of the Korea Healthcare Technology R&D Project, Ministry for Health and Welfare, Republic of Korea (HI10C2020).
Notes
The abstract presented at the "18th Congress of the Asian Pacific Society of Respirology" which will be held on 11-14 Nov 2013 at the Conference Center, Pacifico Yokohama, Japan.
No potential conflict of interest relevant to this article was reported.