A Case of Occupational Hypersensitivity Pneumonitis Associated with Trichloroethylene
Article information
Abstract
Trichloroethylene (TCE) is a toxic chemical commonly used as a degreasing agent, and it is usually found in a colorless or blue liquid form. TCE has a sweet, chloroform-like odor, and this volatile chlorinated organic chemical can cause toxic hepatitis, neurophysiological disorders, skin disorders, and hypersensitivity syndromes. However, the hypersensitivity pneumonitis (HP) attributed to TCE has rarely been reported. We hereby describe a case of HP associated with TCE in a 29-year-old man who was employed as a lead welder at a computer repair center. He was installing the capacitors on computer chip boards and had been wiped down with TCE. He was admitted to our hospital with complaints of dry coughs, night sweats, and weight losses for the past two months. HP due to TCE exposure was being suspected due to his occupational history, and the results of a video-associated thoracoscopic biopsy confirmed the suspicions. Symptoms have resolved after the steroid pulse therapy and his occupational change. TCE should be taken into consideration as a potential trigger of HP. Early recognition and avoidance of the TCE exposure in the future is important for the treatment of TCE induced HP.
Introduction
Trichloroethylene (TCE) is a colorless or blue liquid with a sweet, chloroform-like odor. This volatile chlorinated organic chemical has been widely used in metal fabricating operations, dry cleaning, printing inks, and paints1. Workers can be exposed to TCE through various routes, including inhalation and direct or indirect skin contact, which can result in harmful effects. TCE's toxic effects can manifest in the kidney, liver, neurophysiologic system, and upper respiratory tract. Symptomatically, TCE exposure often results in a cough and sinus congestion2. In addition, several epidemiologic studies have reported an increased risk of cancer with recent exposure to TCE3. Although TCE exposure has been commonly associated with various occupational diseases, it is rarely reported to cause pulmonary parenchymal disease2. However, we report a case of hypersensitivity pneumonitis (HP) in a computer repairman following occupational exposure to TCE.
Case Report
A 29-year-old man was referred to our hospital because of a severe and persistent dry cough, night sweats, and a 10 kg body weight loss over the previous two months. The dry cough and night sweats persisted for two months despite treatment at local outpatient clinic, but he did not complain of exertion-related dyspnea, malaise, or fever upon admission. He was a former smoker, but had stopped smoking five years prior. Notably, he did not have a history of asthma or pulmonary disease. He was not taking any medications or herbal medicines. He had started working in a new occupation of computer repairing 3-4 months ago, and did not use personal protective devices at workplace. Upon physical examination, there were no significant findings, and his vital signs were stable. The chest examination showed clear breath sounds without rales or wheezing, and the cardiac and abdominal examinations were also unremarkable. He had no skin rash or digital clubbing and no musculoskeletal abnormalities. We explored numerous causes for chronic cough, such as upper airway cough syndrome and gastro-esophageal reflux syndrome, but there were no specific findings.
A chest X-ray demonstrated some reticulonodular densities on both upper lobes (Figure 1A). The results of a pulmonary function test were within the normal range, except for mildly reduced diffusion capacity of carbon monoxide (Figure 1B). A chest computed tomography (CT) scan showed tiny ill-defined centrilobular nodules prominent in both upper lobes (Figure 2), which is consistent with HP. Laboratory results were as follows: complete blood count, 9,500/mm3 (neutrophil 71.6%, lymphocyte 22.6%, eosinophil 0.4%); hemoglobin, 14.6 g/dL; platelet count, 286,000/mm3; aspartate aminotransferase/alanine aminotransferase, 13/15 IU/L; total bilirubin, 0.7 mg/dL; total cholesterol, 210 mg/dL; total protein, 7.6 g/dL; blood urea nitrogen, 13.1 mg/dL; and a creatinine of 0.8 mg/dL on biochemistry test. Serum electrolytes were within the normal limits. C-Reactive protein was 0.152 mg/dL (normal range, 0-0.8 mg/dL), and the erythrocyte sedimentation rate was 9 mm/hr (normal range, 0-15 mm/hr). Urinalysis did not reveal proteinuria or glucosuria, but did show microscopic hematuria and pyuria. Serum total IgE level was slightly elevated at 125 kU/L (normal range, <100 kU/L). The panels for autoimmune markers such as antinuclear antibody, rheumatoid factor, anti-cyclic citrullinated peptide antibody, cytoplasmic anti-neutrophil cytoplasmic antibody, perinuclear anti-neutrophil cytoplasmic antibody, anti-Scl 70, anti-SS-A/Ro, and anti-SS-B/La were all within the normal limits.
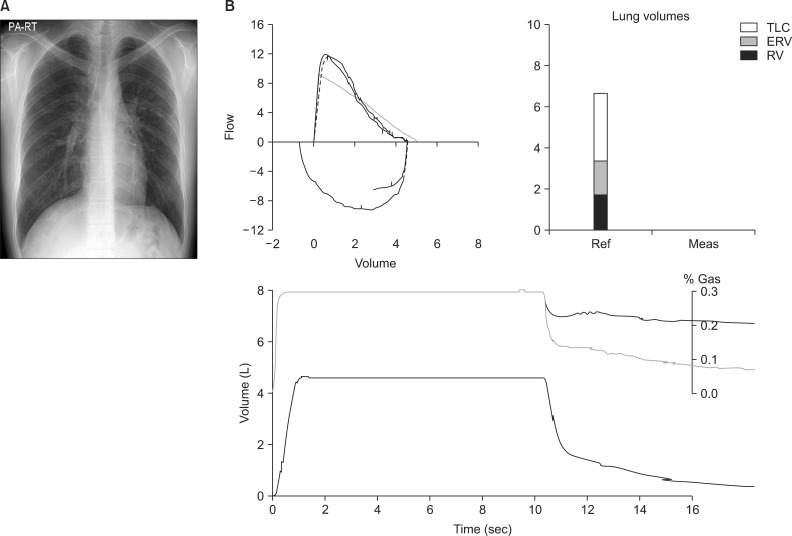
(A) Chest X-ray: some reticulonodular densities on both upper lobes. (B) Pulmonary function test: forced vital capacity (FVC) 4.58 L (90% ref), forced expiratory volume in 1 second (FEV1) 3.87 L (91% ref), FEV1/FVC 84%, FEF25-65% 3.61 L/sec (82% ref), DLCO 23.1 mL/mm Hg/min (83% ref). TLC: total lung capacity; ERV: expiratory reserve volume; RV: residual volume.

Tiny ill-defined nodules, predominant on both upper lobes, suggestive of hypersensitivity pneumonitis.
We performed bronchoalveolar lavage (BAL) and transbronchial biopsy (TBB) in the posterior segment of the right upper lobe and in the anterior-medial segment of the right lower lobe.
BAL revealed the presence of lymphocytes (80%), of which the ratio of CD4 and CD8 was 41.8%:50.6%. TBB showed no pathologic findings.
After gathering details about his occupation, we discovered that his main role in repairing computers was to use lead welding to install capacitors on computer chip boards, and to wipe down the chip boards with TCE. His duties were performed without personal protective equipment or a proper ventilation system in the workplace. Unfortunately, we could not test his serum or urine TCE level, but his lead level in serum was 1.67 µg/L (normal range, <20 µg/L) and in urine was 6.24 µg/L (normal range, <80 µg/L), which were both within the normal range.
With this information, we strongly suspected that the HP was related to TCE exposure. We recommended him to change the occupation, but he could not find alternative occupation due to economic status. We started treatment with 30 mg oral prednisolone daily, and after oral prednisolone treatment, the dry cough was slightly improved, but it was not entirely resolved. For a more accurate diagnosis, he underwent surgical lung biopsy via video-associated thoracoscopy (VATS). Pathologic findings were present, which consisted of multifocal, patchy, fibroblastic proliferations, chronic inflammatory cell infiltration around the respiratory bronchiole, and associated foamy histiocytic aggregation (Figure 3). With these biopsy results under the consideration of both clinical presentation and radiological findings, we reached a final diagnosis of subacute HP. After receiving the diagnosis, he returned to work, but revisited the outpatient clinic due to worsening, severe cough and anterior chest pain. Chest CT was performed and showed pneumomediastinum and slightly aggravated HP. He was exposed to TCE repeatedly after discharge from the hospital, and we think the pneumomediastinum may have been developed by aggravation of symptoms including severe cough. He was treated with high concentration oxygen and steroid pulse therapy (methylprednisolone 62.5 mg bid I.V.), but despite this, recurrent pneumothorax developed. He underwent wedge resection for two subpleural bullae at the right apex, and he underwent a thoracoscopic pleurectomy to treat the recurrent pneumothorax. After the operation, the recurrent pneumothorax resolved, but his chronic cough still remained after the steroid treatment. We recommended again strongly to change occupation, and after entering a new department, we tapered down the oral prednisolone slowly until discontinuation. After changing occupation and finishing the steroid regimen, the symptoms improved and the CT findings were almost completely resolved (Figures 4, 5).
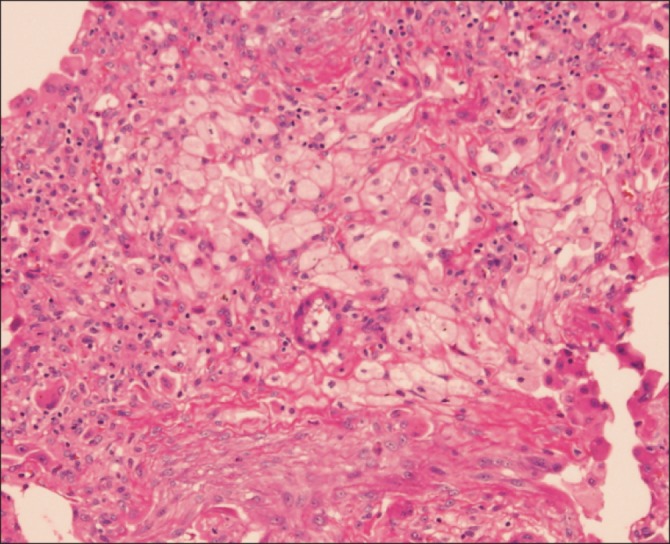
Multifocal patchy young fibroblastic proliferation, chronic inflammatory cell infiltration around respiratory bronchiole associated with foamy histiocytic aggregations (H&E stain, ×100).
Discussion
TCE is a colorless or blue liquid with a sweet, chloroform-like order. This volatile, chlorinated, organic chemical is often used as a degreaser for metal parts or as a general-purpose solvent for lipophilic compounds. It is also an ingredient in paint removers, typewriter correction fluids, rug-cleaning fluids, and spot removers4. TCE can be absorbed cutaneously, through the digestive tract, or via respiratory routes, and it distributes throughout the body, but goes preferentially to fat. TCE is metabolized primarily in the liver to its active forms, trichloracetic acid and free trichloroethanol, and TCE is eliminated through the kidneys and lungs. Acute and chronic inhalation exposure to TCE from industrial degreasing operations can affect the human central nervous system, resulting in dizziness, headache, confusion, euphoria, facial numbness, and weakness. In addition, liver, kidney, immunological, endocrine, developmental, and carcinogenic effects have been reported in humans5. Nonetheless, TCE is commonly used at many workplaces because of its excellent solubility for grease and fat, its volatility, incombustibility, and its economic value.
Several reports revealed occupational TCE to be a cause of idiosyncratic generalized skin disorders and toxic hepatitis6. In addition, Goon et al.7 defined TCE hypersensitivity syndrome, which includes exfoliative dermatitis, mucous membrane erosion, eosinophilia, and hepatitis. However, Tanios et al.2 published the only report of HP associated with TCE, which was observed in a 42-year-old woman working as a dry cleaner. In this case, her role in the workplace was limited to removing spots from clothing with TCE. In addition to her occupational history, the diagnosis of HP due to TCE exposure was further confirmed by TBB. Like the gentleman in our case, she also improved with 40 mg oral prednisone and a change of career.
HP is defined as a pulmonary disease with symptoms of dyspnea and cough caused by inhalation exposure to a large variety of antigens of which the patient has been previously sensitized8. HP can be divided clinically into acute, subacute, and chronic types. Acute HP develops within 4-8 hours of the exposure to triggering antigens, whereas the subacute and chronic forms present progressively as dyspnea, weight loss, and cough. Early diagnosis and effective correction of the cause is very important because chronic HP, or a diagnosis in its advanced stages, can lead to disability and is associated with increased mortality9. However, there is no one precise diagnostic criterion, although many diagnostic criteria recommendations have been published8,9. One good example was published by Lacasse et al.10, who described the following significant predictors of HP: 1) exposure to a known offending antigen, 2) positive precipitating antibodies, 3) recent episode of symptoms, 4) inspiratory crackles, 5) symptoms 4-8 hours after exposure, and 6) weight loss10. The probability of HP is 98% when all six predictors are present and 0% when none of the predictors are identified. However, variable diagnostic tests such as laboratory study, chest radiography, and lung biopsy are usually performed if HP is highly suspected through a detailed history. However, when diagnosing, it is important that HP can be distinguished from sarcoidosis and other interstitial lung disease. After a correct, early diagnosis and effective avoidance of the cause, HP is treated by oral or systemic corticosteroids. Some studies suggest that inhaled steroid11 or pentoxifylline12 may be an effective treatment and that systemic corticosteroids could be reserved for severe cases or when the offending antigen cannot be completely removed.
In our case, we could differentiate HP from sarcoidosis because no granulomatous lung lesions were found on VATS biopsy, and because of the inverse ratio of CD4/CD8 on BAL fluid analysis. We could also distinguish HP from connective tissue-related interstitial lung disease because there were normal levels of autoimmune markers. Additionally, we suspected HP rather than interstitial lung disease based on the subject's detailed occupational history. The symptoms appeared after 1-2 months from the initiation of the computer repairing work, and on chest CT, there were tiny centrilobular nodules without honeycombing change. Upon pathological findings, inflammatory cell infiltration and histiocytic aggregation were shown in this case, whereas in typical HP, noncaseating granulomas, monuclear cell infiltration in bronchiole and foamy histiocytes in interstitium are present13. We cannot completely exclude the possibility of viral infection only by pathological findings, but considering the clinical presentation and radiological findings, we could make a diagnosis of subacute HP. He was treated with oral prednisolone, but experienced a recurrence of the cough because he was reinstated to his former position without environmental remediation or personal protective equipment. Finally, after changing his workplace department, he improved.
We report that TCE exposure can result in HP. Early identification and remediation of the triggering source can prevent disability or serious complications. In conclusion, if HP develops in computer repairman, we should consider TCE as a likely triggering or offending cause.

