Recent Trends of Lung Cancer in Korea
Article information
Abstract
Lung cancer is one of the leading causes of cancer-related deaths in Korea. Although the smoking rate has decreased over time, the prevalence of lung cancer still remains high. In this study, we reviewed recent trends on the incidence, epidemiology, screening, diagnosis, and treatment of lung cancer in Korea by analyzing data from the national lung cancer registry and recently-published studies. Although approximately 40% of patients with non–small cell lung cancer (NSCLC) were diagnosed as stage IV, the 5-year relative survival rate improved from 11.3% (1993–1995) to 30.2% (2013–2017), possibly due to advances in methods of diagnosis and therapy. In addition, the 2019 implementation of the national lung cancer screening program with low-dose computed tomography may have also contributed to these improvements in survival rates. Recently, molecular diagnosis has become more widely used in the identification of genetic mutations in tissue specimens. Target therapy and immune checkpoint inhibitors have also been successfully used, particularly in cases of advanced NSCLC. In the future, further research on the optimal management of lung cancer remains necessary.
Introduction
Lung cancer is the most common cancer worldwide in terms of both incidence and mortality [1]. The situation is similar in Korea, where the crude incidence of lung cancer was the third highest among all cancers, according to the annual report of the Korean National Cancer Registration Statistics. In addition, lung cancer is the leading cause of cancer-related death in Korea [2]. A national lung cancer screening program using low-dose computed tomography was recently launched in Korea [3], and various treatments such as target therapy and immune checkpoint inhibitors have been developed for lung cancer [4,5]. Based on recent nationwide data, we have briefly reviewed the epidemiology, screening, diagnosis, and treatment of lung cancer in Korea.
Epidemiology
According to the Korea Central Cancer Registry, lung cancer was the third most common cancer (11.6%, 26,985 patients: 69% were male, and 31% were female) in 2017 after stomach and colon cancers [6]. The overall crude incidence rate was 52.7 per 100,000 population, and the age-standardized lung cancer incidence rate age-adjusted to the Korean standard population (Korean age-standardized incidence rate, KASIR) was 27.5 per 100,000 population in 2017. The crude incidence and KASIR of lung cancer were higher in male patients (72.9 per 100,000 and 42.7 per 100,000, respectively) than in female patients (32.5 per 100,000 and 15.8 per 100,000, respectively) (Figure 1) [6]. The incidence of lung cancer increased as both men and women aged, especially from the age of 65 years onwards. The KASIR of lung cancer also increased significantly with age and reached a peak at 80–84 years for men and a peak at 85 years for women [6].

Trends in crude incidence rates and age-standardized lung cancer incidence rates per 100,000 in the Korean population: overall (A), men (B), and women (C).
Until 2010, the most frequent histological type was squamous cell carcinoma in Korea. However, since 2011, adenocarcinoma has been the most commonly diagnosed cancer [7]. These changes in the histology of lung cancer in Korea are the same as those observed for the global trend [8]. Additionally, adenocarcinoma in women is being diagnosed at a high rate, and this rate is increasing [9,10]. Especially in Asia, most female lung cancer patients are not smokers, and their histologic type is adenocarcinoma [11]. In addition, as observed for squamous cell carcinoma, the incidence of small cell carcinomas is decreasing (Figure 2) [12].

Trend in percent change in histological subtypes of lung cancer in Korea: overall (A), men (B), and women (C).
At the time of diagnosis of lung cancer, the proportions of non–small cell lung cancer (NSCLC) patients in each clinical stage were as follows: 25.6% in stage I, 9.5% in stage II, 22.9% in stage III, and 42.0% in stage IV (Figure 3) [13]. The prognosis of lung cancer differs according to the clinical stage, and the 5-year relative survival rate (2013–2017) was 69.0% for localized cancer, 39.3% for regional cancer, 7.7% for distant cancer, and 22.4% for unknown stage cancer [6].
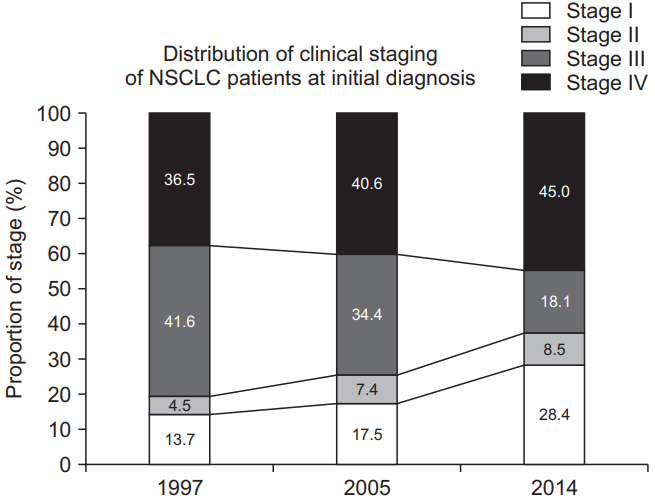
Distribution of clinical staging of non–small cell lung cancer (NSCLC) patients at initial diagnosis.
In 2017, the 5-year relative survival rate for lung cancer in Korea improved compared with the rate observed more than 10 years ago. Overall, the 5-year relative survival rate of lung cancer in Korea increased from 16.5% (2001–2005) to 30.2% (2013–2017), with an increase from 15.3% (2001–2005) to 25.2% (2013–2017) in male patients and from 20.1% (2001–2005) to 41.5% (2013–2017) in female patients (Figure 4) [6]. The 5-year relative survival rate increase might be due to the reduction in the smoking rate [14], advances in screening using imaging tools [15,16], and the development of new chemotherapy approaches such as target therapy [17].
Screening
The National Lung Screening Trial, which was conducted in the United States and used low-dose chest computed tomography (CT) for high-risk smokers, showed a 20% decrease in lung cancer mortality and 7% decrease in overall mortality, with a similar result in Europe [15,16,18]. Based on these results, Korea conducted the Korean Lung Cancer Screening Project (K-LUCAS). As a result, among the K-LUCAS participants, 79(0.58%) were diagnosed with lung cancer, and among these 79, 54 of patients (68.4%) diagnosed early stage (stage I or II). This was evaluated to be three times higher than the early lung cancer diagnosis rate (21%) among all registered lung cancer patients in Korea [3]. Thus, from August 2019, the world’s first national health examination project was launched to detect lung cancer early through low-dose chest CT for high-risk smokers between 55 and 74 years of age, who smoked more than 30 pack-years.
Diagnosis
Imaging methods, such as CT and positron emission tomography–computed tomography, are necessary for the diagnosis and staging of lung cancer. In addition, bronchoscopy, CT-guided percutaneous needle aspiration (PCNA), and percutaneous needle biopsy (PCNB) are traditionally performed for the histologic diagnosis of lung cancer. Recently, endobronchial ultrasound bronchoscopy (EBUS) has made it easier to perform a biopsy on lung cancer lesions that are difficult to access with CT-guided PCNA and PCNB for central lung lesions [19]. Radial probe endobronchial ultrasound or navigation bronchoscopy has been introduced for peripheral lung lesions in an inaccessible position for EBUS–transbronchial needle aspiration, and it can replace CT-guided PCNA for biopsy [20-22]. Furthermore, a liquid biopsy method has been recently developed to detect circulating cell-free tumor DNA in the body fluid and blood of patients using quantitative realtime polymerase chain reaction, digital polymerase chain reaction, and next-generation sequencing (NGS). Liquid biopsy is expected to be used as a diagnostic tool for lung cancer in the near future (Table 1) [23].
Recently, molecular diagnosis has been implemented to identify mutant epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK), ROS1 , and BRAF , which might affect treatment and clinical outcome (Table 2) [24]. Korea has a high percentage of EGFR mutations in adenocarcinoma (overall 29% to 50%), similar to other Asian countries (overall 47%) [25,26]. Additionally, for women who are non-smokers in Korea, the rate of EGFR mutations in adenocarcinoma is particularly high [27]. These facts confirm that molecular diagnostics for lung cancer play a large role in the treatment of lung cancer in Korean women who have no smoking history. In the molecular diagnosis of lung cancer, the peptide nucleic acidmediated clamping method is one of the most commonly used methods for detecting gene mutations in cancer tissue specimens in Korea [4,28]. Since 2017, an NGS technology-based genetic panel test has been designated for national health insurance benefits in Korean lung cancer patients, and it will be able to quickly identify mutant EGFR, ALK, ROS , and BRAF [29].
Treatment
There are three main treatments for lung cancer: surgery, radiation therapy, and chemotherapy. Treatment may vary depending on the histological findings, the stage of the disease, and the patient’s condition. A previous study showed that 37.6% of patients initially diagnosed with NSCLC, regardless of stage, underwent surgery including adjuvant therapy; 8.3% of patients underwent radiation therapy only; 4.2% of patients underwent concurrent chemoradiotherapy (CCRT); 29.0% of patients received chemotherapy; and 12.8% of patients were provided supportive care without chemotherapy. 8.1% of patients were unknown (Figure 5) [26].
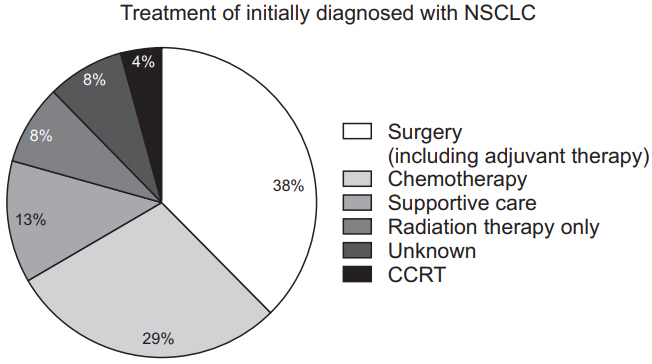
Current treatment of initially diagnosed with non–small cell lung cancer (NSCLC) in Korea. CCRT: concurrent chemoradiation therapy.
Target therapy and immune checkpoint inhibitors (ICIs) have recently been administered for treatment, especially in advanced NSCLC (Table 2) [5,30]. According to one study, adenocarcinoma involves EGFR mutations in ~50% of the cases; therefore, in Korea, EGFR should be tested, especially in adenocarcinoma [31]. In Korea, the Ministry of Food and Drug Safety has approved first-generation EGFR inhibitors (gefitinib and erlotinib) and second-generation EGFR inhibitors (afatinib and dacomitinib) as first-line therapy for adenocarcinoma after confirmation of EGFR mutation. When cancer proceeds despite these treatments, testing is performed for T790M mutation, and if found, osimertinib is recommended for therapy [32]. In addition, crizotinib, alectinib, and ceritinib, which are currently approved for use in Korea, can be considered as primary treatments when ALK mutations are identified (~5% of NSCLC patients) [33,34]. Recently, the use of brigatinib was approved in Korea as first-line therapy in patients with NSCLC with ALK mutation. Crizotinib has been approved as first-line therapy in Korea for cases in which ROS1 mutation is confirmed. Additionally, the use of a combination of dabrafenib and trametinib has been approved in Korea when there is a BRAF V600E mutation. ICIs—programmed cell death-1 protein (PD-1)/programmed dealth-ligand 1 (PD-L1) inhibitors such as nivolumab, pembrolizumab, atezolizumab, and durvalumab—can be used when the presentation of PD-1/PD-L1 is confirmed in advanced NSCLC [35]. Nivolumab, pembrolizumab, and atezolizumab have been approved in Korea as salvage treatments for lung cancer patients who failed prior platinum-based chemotherapy. However, the approval conditions for administration are as follows: pembrolizumab is administered when PD-L1 expression ≥50% in lung cancer, nivolumab is administered when PD-L1 expression ≥10% in lung cancer, and atezolizumab is approved for administration irrespective of the level of PD-L1 expression in lung cancer. Durvalumab has been approved in Korea as consolidation therapy in patients with unresectable lung cancer of stage III with PD-L1 expression over 1%, especially in patients without disease progression after CCRT.
Conclusion
Lung cancer has the highest mortality rate among cancers worldwide. For this reason, among all countries, Korea was the first to conduct a national lung cancer screening program for high-risk lung cancer patients. Efforts are under progress to diagnose lung cancer more easily and quickly by introducing new diagnostic technologies. Additionally, precision medicine has been introduced, and many clinical trials are ongoing. Further large-scale studies are needed to investigate the optimal management of patients with lung cancer.
Notes
Authors’ Contributions
Concept and design of the study: Lee GK. Kim HC, Choi CM. Manuscript writing: Lee GK, Kim HC. Writing - original draft preparation: Lee GK. Writing - review and editing: Kim HC, Choi CM. Approval of final manuscript: all authors.
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Funding
No funding to declare.



