Cardiac Dysfunction Is Not Associated with Increased Reintubation Rate in Patients Treated with Post-extubation High-Flow Nasal Cannula
Article information
Abstract
Background
Cardiac dysfunction patients have long been considered at high risk of reintubation. However, it is based on past studies in which only conventional oxygen therapy was applied after extubation. We investigated association between cardiac dysfunction and reintubation rate in situation where high-flow nasal cannula (HFNC) was widely used during post-extubation period.
Methods
We conducted a retrospective observational cohort study of patients treated with HFNC after planned extubation in medical intensive care unit of single tertiary center. Patients were divided into normal function group (ejection fraction [EF] ≥45%) and cardiac dysfunction group (EF <45%). The primary outcome was reintubation rate within 72 hours following extubation.
Results
Of 270 patients, 35 (13%) had cardiac dysfunction. Baseline characteristics were similar in both groups. There were no differences in the changes in vital signs between the two groups during the first 12 hours after extubation except diastolic blood pressure. The reintubation rates were 20% and 17% for cardiac dysfunction group and normal function group, respectively (p=0.637). In a multivariate Cox regression analysis, cardiac dysfunction was not associated with an increased risk of reintubation within 72 hours following extubation (hazard ratio, 1.56; p=0.292).
Conclusion
Cardiac dysfunction was not associated with increased reintubation rate within 72 hours when HFNC is immediately applied after planned extubation.
Introduction
High-flow nasal cannula (HFNC) is increasingly used in critically ill patients, because it has a number of advantages over conventional oxygen therapy (COT) or non-invasive ventilation (NIV) including favorable physiologic parameters, ease of use, and patient comfort [1,2]. It is mainly indicated for patients with acute hypoxemic respiratory failure [3,4], but can also be applied to various conditions including post-extubation [5-7].
HFNC has been demonstrated to be superior to COT in terms of reducing reintubation rate and post-extubation respiratory failure in patients with low risk for reintubation [6]. However, only a few patients meet the low risk criteria because intensive care unit (ICU) patients are getting older and often have multiple comorbidities [8]. Recently, two randomized trials compared the efficacy of HFNC on reintubation with NIV or NIV plus HFNC in high risk patients. HFNC was non-inferior to NIV but inferior to NIV plus HFNC as preventive measures in post-extubation period [9,10]. Based on these results, applying NIV during post-extubation period may be advisable in high risk patients. Indeed, current guidelines recommend preventative NIV after extubation in high risk patients though the strength of recommendation or the degree of evidence differs [11,12]. However, given that most of medical ICU patients belong to high risk group and NIV has several drawbacks such as poor tolerance, cost, and need for trained staff [13], it is difficult to apply NIV to every high risk patients in practice. Moreover, high risk group was defined differently in each study, and it was composed of heterogeneous patients. Therefore, it seems reasonable to refine and narrow down the high risk group.
Past studies found that cardiac dysfunction, either as a cause of respiratory failure or as an underlying disease, harbors increased risk of reintubation [14,15]. However, in those studies, COT was applied after extubation, so the situation is different from the current increasing use of post-extubation HFNC [16]. We planned this study to investigate association between cardiac dysfunction and reintubation rate within 72 hours in situation where HFNC was widely used during post-extubation period.
Materials and Methods
1. Study design and population
We conducted a retrospective observational cohort study of patients treated with HFNC after planned extubation in medical ICU at Korea University Guro Hospital between January 2017 and December 2019. This study was approved by the Institutional Review Board (2020GR0518). The need for informed consent was waived because of the observational nature of the study. Adult patients who were admitted to the medical ICU and mechanically ventilated for ≥48 hours, passed a spontaneous breathing trial (SBT), and were supported by HFNC immediately after extubation were included in the study. Patients were excluded according to the following criteria: application of COT or NIV immediately after extubation; no planned extubation; and no echocardiography data during the ICU stay.
2. Weaning and post-extubation practice
Patients ventilated for >24 hours underwent a spontaneous awakening trial, and if successful, they were evaluated for the possibility of weaning according to the institutional protocol. A patient underwent SBT with 8–10 cm H2O pressure support and 6 cm H2O positive end-expiratory pressure when the condition leading to intubation had been improved, and hemodynamic and respiratory stabilities were achieved. If a patient passed SBT, extubation ensued. After planned extubation, HFNC (Airvo 2, Fisher and Paykel Healthcare, Laval, QC, Canada) was initiated at a flow rate of 50 L/min with fraction of inspired oxygen (FiO2) of 0.5. Detailed protocol of weaning and post-extubation practice was reported in elsewhere [17].
3. Data collection
Data were collected retrospectively for all patients commencing HFNC after extubation during the study period. To reduce bias, well-trained research nurses collected data from the electronic medical records (EMRs) using standardized data collection protocols, and patients with >20% missing data for each variable were excluded from the analysis. Baseline characteristics including demographic features, and clinical and laboratory findings at the time of admission and extubation were collected. Physiologic parameters and HFNC settings (flow rate and FiO2) were serially recorded after extubation. For patients who required reintubation, the time from extubation to reintubation was documented, and the reason for reintubation was described. The primary outcome was reintubation rate within 72 hours following extubation. Secondary outcomes included the time to reintubation, the reason for reintubation, ICU length of stay (LOS), ICU mortality, and hospital mortality.
4. Cardiac dysfunction
In our practice, formal echocardiography has been routinely performed within 3 working days after ICU admission. An echocardiographer carried out the examination, and cardiologists confirmed the result; neither were involved in this study. The ejection fraction (EF) was measured by modified Simpson method. Cardiac dysfunction was defined as EF <45% [18]. Eligible patients were divided into normal group (EF ≥45%) and cardiac dysfunction group (EF <45%).
5. Statistical analysis
Continuous variables are reported as the median with interquartile range, and categorical variables as the number and percentage. Baseline characteristics and clinical outcomes were compared between the two groups using Fisher’s exact test and the Mann–Whitney U test, as appropriate. We compared changes in vital signs and HFNC settings over the first 12 hours after extubation using a generalized linear model. The cumulative rate of reintubation was assessed by Kaplan-Meier curve and compared using the log-rank test. To investigate factors associated with reintubation within 72 hours following extubation, Cox proportional hazard model was used. Variables with p<0.05 in univariate analysis and cardiac dysfunction were incorporated in multivariate analysis. The results were reported as hazard ratio (HR) of each variable with 95% confidence intervals (CI). Data were analyzed using SPSS version 20.0 (IBM Corp., Armonk, NY, USA) and GraphPad Prism version 9.0 (GraphPad Software, San Diego, CA, USA). All tests were two-sided, and p<0.05 was taken to indicate statistical significance.
Results
During the study period, 880 patients were treated with mechanical ventilation in the medical ICU. Of these patients, 222 were excluded because they failed to proceed to planned extubation; 52 patients were excluded because of insufficient EMRs; 288 were excluded because they were treated with COT after extubation; and 42 were excluded because they were treated with NIV after extubation. Therefore, 276 patients were included in the study. As EF data were not available for six patients, 270 were included in the final analysis (Figure 1). The median follow-up period was 22 days.
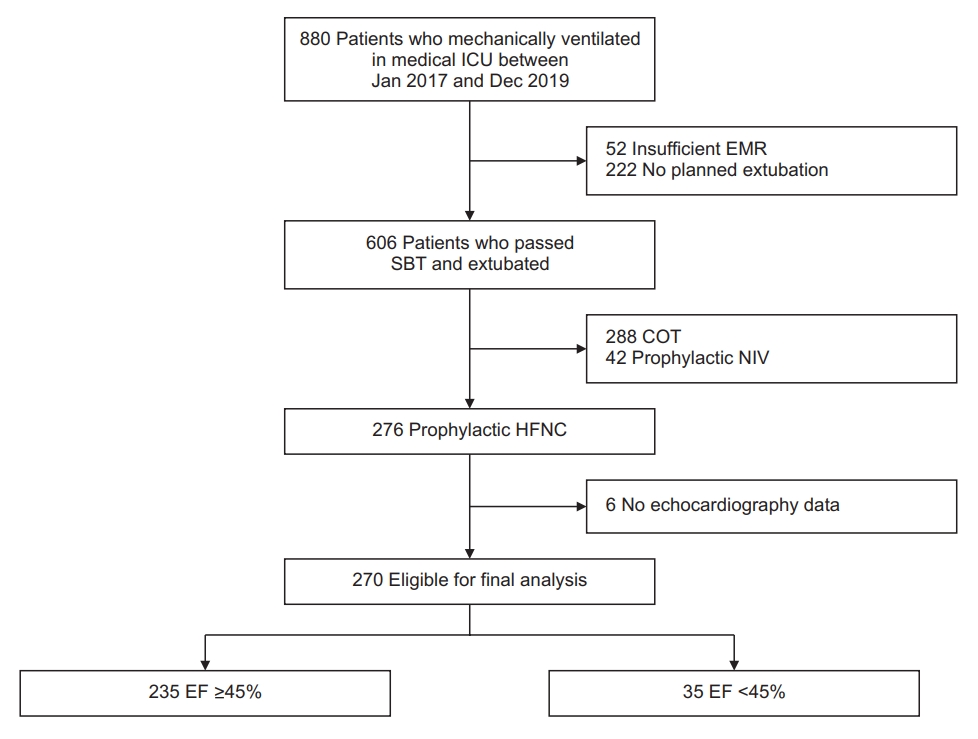
Flow chart of enrolled patients. ICU: intensive care unit; EMR: electronic medical record; SBT: spontaneous breathing trial; COT: conventional oxygen therapy; NIV: non-invasive ventilation; HFNC: high-flow nasal cannula; EF: ejection fraction.
1. Baseline characteristics
Overall, there were no differences in baseline characteristics between the two groups (Table 1). On the day of extubation, patients in the cardiac dysfunction group had higher rates of treatment with vasoactive agents, particularly dobutamine, compared to the normal function group (45.7% vs. 16.2%, p<0.001). Renal replacement therapy was applied more frequently in the cardiac dysfunction group than the normal function group, although the difference was not significant (20% vs. 9.4%, p=0.076). The degree of organ dysfunction, gas exchange, and vital signs were similar in both groups. Oxygenation indices, including partial pressure of oxygen (PaO2) and the PaO2/FiO2 ratio, were higher in the cardiac dysfunction group (121 mm Hg vs. 103 mm Hg, p=0.009; 373 vs. 286, p=0.001). The abnormal cardiac findings of each group are shown in Supplementary Table S1.
2. Group comparison of changes in physiologic parameters and HFNC settings
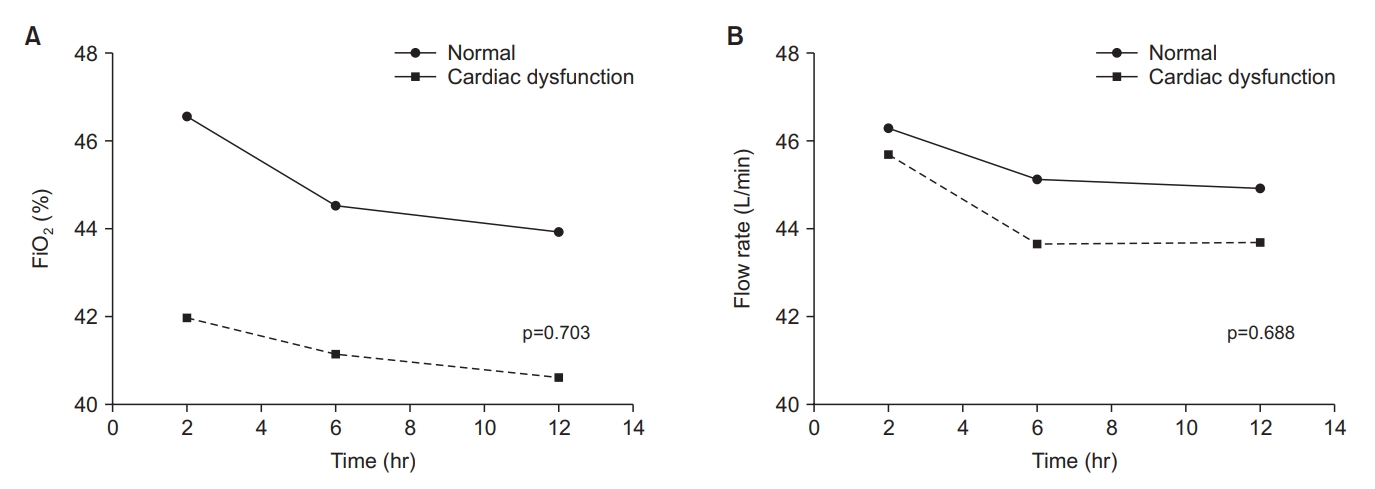
There were no differences in the changes in vital signs between the two groups during the first 12 hours after extubation except diastolic blood pressure (Figure 2). There was also no group difference in changes in flow rate and FiO2, which tended to decrease over time in both groups (Figure 3).
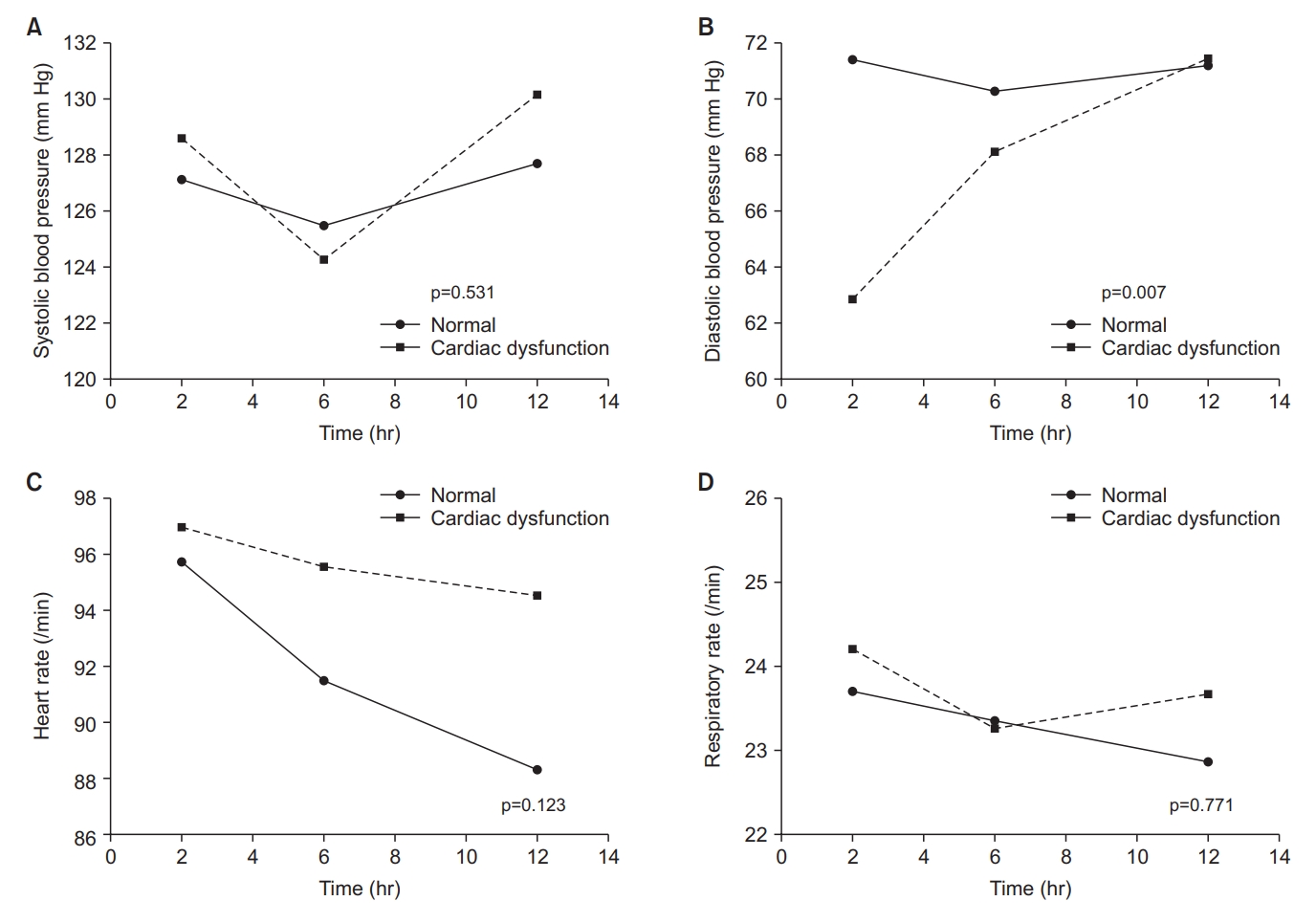
Group comparison of changes in physiologic parameters within 12 hours after extubation. (A) Systolic blood pressure change within 12 hours after extubation. (B) Diastolic blood pressure change within 12 hours after extubation. (C) Heart rate change within 12 hours after extubation. (D) Respiratory rate change within 12 hours after extubation.
3. Clinical outcomes
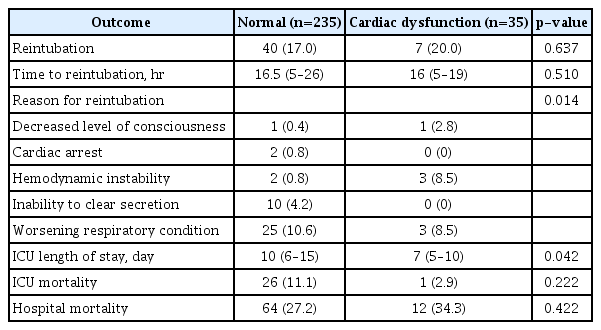
The clinical outcomes in the cardiac dysfunction and normal function groups are presented in Table 2. The rate of reintubation was not different between the two groups (20% vs. 17%, p=0.637), but the main reason for reintubation was different. In the normal function group, worsening respiratory condition (10.6%) and inability to clear secretion (4.2%) were common causes of reintubation. In the cardiac dysfunction group, worsening respiratory condition (8.5%) and hemodynamic instability (8.5%) were common causes (p=0.014). The median ICU LOS was shorter in the cardiac dysfunction group (7 days vs. 10 days, p=0.042). ICU and hospital mortalities were higher in normal and cardiac dysfunction group, respectively without statistical significance. The median time to reintubation was not different between the two groups (16 hours vs. 16.5 hours, p=0.510), and most cases of reintubation occurred within the first 24 hours (Supplementary Figure S1). The cumulative rate of reintubation was depicted by the Kaplan-Meier curve (Figure 4). The results of Cox regression analysis were given in Table 3. The presence of cardiac dysfunction was not associated with an increased risk of reintubation within 72 hours following extubation (HR, 1.56; 95% CI, 0.683–3.558; p=0.292).
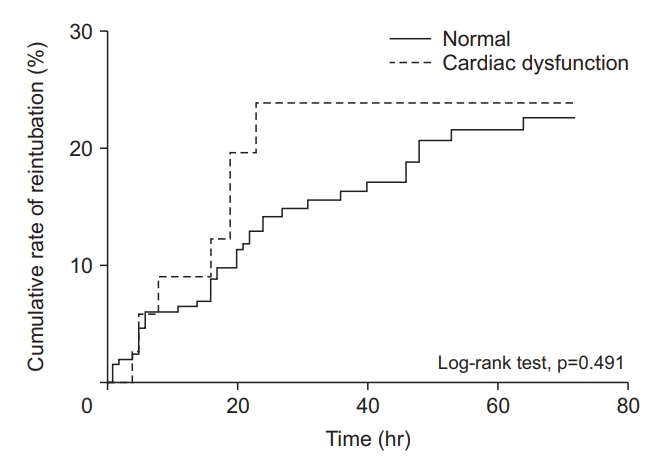
Kaplan-Meier plots showing the cumulative rate of reintubation within 72 hours after extubation according to the presence of cardiac dysfunction.
Discussion
In this study, we categorized patients into normal and cardiac dysfunction groups based on EF, and compared reintubation rate under condition of HFNC following planned extubation. Interestingly, it was not higher in patients with cardiac dysfunction, which is established risk factor of reintubation. In addition, clinical outcomes and physiologic responses after extubation were similar between two groups.
For patients with cardiac dysfunction, positive pressure ventilation provides advantages of lowering left ventricular preload [19]. Because extubation eliminates these benefits, it could lead to pulmonary edema and respiratory failure. However, HFNC can supply inspiratory pressure based on flow rate. Roca et al. [20] reported that HFNC reduced inspiratory collapse of inferior vena cava (IVC) in patients with heart failure, indicating a decrease in preload. In addition, when the flow rate was increased from 20 L/min to 40 L/min, the inspiratory collapse of IVC was significantly reduced from 28% to 21% [20]. In our study, the median flow rate was about 45 L/min, which might be effective to prevent the increase in preload following extubation. Of note, all reintubation events occurred within first 24 hours in the cardiac dysfunction group. These findings might reflect rapid response to elimination of positive pressure in those patients who were greatly affected by positive pressure. However, there was no statistical significance, and few studies examined the relation of cause of extubation failure, its onset time, and patient characteristics.
In previous studies, patients at high risk of reintubation were heterogeneous, so the effectiveness of NIV may vary depending on patients’ characteristics. Ferrer et al. [21] reported that NIV was superior to COT in terms of respiratory failure and mortality, but there was no difference in reintubation rate, and a mortality benefit was found in patients with hypercapnia. In another study demonstrating the efficacy of NIV in high risk patients, about one third of patients had persistent hypercapnia [22]. A recent trial showed that NIV tended to work better in hypercapnic patients [10]. In contrast, Chang et al. showed that the reintubation rate was not different between heart failure patients treated with NIV or HFNC. Notably, the number of chronic obstructive pulmonary disease patients was small in that study [23]. In our study, carbon dioxide concentration of the two groups was in the normal range. Based on these findings, post-extubation NIV is helpful for hypercapnic patients. However, it does not seem essential for patients with cardiac dysfunction, who are normocapnic and supported with HFNC.
Although this study suggested the utility of post-extubation HFNC in patients with cardiac dysfunction, several limitations should be acknowledged. First, cardiac dysfunction was the accompanying characteristics, neither the primary diagnosis nor the cause of respiratory failure. In several studies, patients were regarded as high risk group when cardiac failure was the primary indication for mechanical ventilation [9,14,21]. Therefore, caution should be taken when applying our findings to such patients. Second, EF was not measured at the time of SBT or extubation. It would be better to understand the association between cardiac function and reintubation risk if EF was measured at that moment. However, unlike previous work that did not present EF measurement time [10,15,22], we provided EF near the time of SBT or extubation, reflecting cardiac function more precisely. Third, respiratory mechanics such as rapid shallow breathing index or maximal inspiratory pressure were not compared or included in the analysis though they were well-known weaning predictors. However, SBT was performed according to the institutional protocol in which tidal volume and respiratory are included [17], we believe that respiratory rate, tidal volume, and eventually rapid shallow breathing index would be in adequate range in both groups. In addition, this study was conducted retrospectively at a single institution in a small number of medical ICU patients. Therefore, one should be cautious to generalize our findings, and sample size would be not enough to detect statistical significance. As is well known, retrospective study is not free from bias. In patients with cardiac dysfunction, only those with a less severe condition may have been included in the study; severe cases may have been treated with NIV or tracheostomized. In fact, ICU LOS was shorter, and ICU mortality was lower in cardiac dysfunction group, although the differences were not statistically significant. Oxygenation indices were also better in cardiac dysfunction group. On the other hand, there were no differences in age, comorbidities, severity at admission, or degree of organ dysfunction on the day of extubation between the two groups. Renal failure was more prevalent in cardiac dysfunction group. In addition, the number of patients with NIV support following extubation was small during the study period. Patients in the cardiac dysfunction group were treated more frequently with dobutamine on the day of extubation. Use of dobutamine may have reduced the difference in EF between the two groups and helped to overcome hemodynamic burden following extubation. But, in Cox regression analysis, dobutamine use was not associated with increased reintubation rate (Table 3). Finally, we did not directly compare HFNC and NIV. However, as the reintubation rate did not differ between the normal function and cardiac dysfunction groups, we judged that cardiac dysfunction is not significant risk factor when HFNC is applied immediately after extubation.
In conclusion, cardiac dysfunction was not associated with increased reintubation rate within 72 hours when HFNC is immediately applied after planned extubation. Our findings suggest the possibility that risk factors of reintubation may differ depending on which modality are applied. Further research is required to identify and reassess risk factors of reintubation.
Notes
Authors’ Contributions
Conceptualization: Sim JK, Lee YS. Methodology: Lee YS. Validation: Min KH, Hur GY, Lee SY, Shim JJ. Formal analysis: Sim JK. Data curation: Sim JK, Choi J, Oh JY. Writing - original draft preparation: Sim JK. Writing - review and editing: Sim JK, Lee YS. Approval of final manuscript: all authors.
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Funding
No funding to declare.
Supplementary Material
Supplementary material can be found in the journal homepage (http://www.e-trd.org).
Abnormal cardiac findings.
Reintubation rate at intervals of 24 hours within the 72 hours after extubation.