An Open-Label, Multicentre, Observational, Post-Marketing Study to Monitor the Safety and Effectiveness of Umeclidinium/Vilanterol in Korean Patients
Article information
Abstract
Background
Umeclidinium/vilanterol (UMEC/VI; ANORO ELLIPTA, GSK) is a commonly used dual bronchodilator. This study evaluated the safety and effectiveness of UMEC/VI in Korean patients with chronic obstructive pulmonary disease (COPD) over a 6-year period.
Methods
This was an open-label, multicentre, observational, post-marketing surveillance study. A total of 3,375 patients were enrolled consecutively in 52 hospitals, by 53 physicians, between July 2014 and July 2020. Patients who were administered UMEC/VI (fixed-dose 62.5 μg/25 μg) at least once and were monitored for safety and effectiveness were included in the analysis. Incidence and severity of adverse events (AEs) reported after administrating at least one dose of UMEC/VI were monitored, including unexpected adverse events (UAEs) and adverse drug reactions (ADRs). Effectiveness of UMEC/VI after 24 weeks of administration was also assessed using physician’s evaluation (effective, ineffective/no change, worsening, indeterminable) and lung function improvement.
Results
Of 3,375 patients, 3,086 were included in the safety assessment group (mean age±standard deviation: 69.76±8.80 years; 85.9% male [n=2,652]; 73.1% aged ≥65 years [n=2,255]). The overall incidence of AEs was 28.8% (n=890), of which 2.2% (n=67) were ADRs. Serious AEs and UAEs were reported in 181 (5.9%) and 665 (21.6%) patients, respectively, and two patients (<0.1%) reported unexpected severe ADR. Of the 903/3,086 patients analysed for effectiveness, most (82.8%, n=748) showed overall disease improvement after UMEC/VI treatment.
Conclusion
This study confirmed UMEC/VI administered to Korean patients according to the prescribing information was well-tolerated and can be considered an effective option for COPD treatment.
Introduction
Chronic obstructive pulmonary disease (COPD) is a preventable and treatable disease characterized by airflow limitation that is not fully reversible. This limitation is primarily due to small airway disease and parenchymal destruction associated with an abnormal inflammatory response of the lungs, mainly caused by cigarette smoking [1]. COPD is a major cause of ill-health, characterized by chronic and progressive breathlessness (dyspnea), cough, and sputum production [2], and is one of the top three causes of death worldwide [2-5].
Bronchodilator therapy can ameliorate COPD symptoms, reduce the frequency and severity of exacerbations, and improve health status and exercise tolerance [2,6]. Umeclidinium/vilanterol (UMEC/VI; ANORO ELLIPTA, GSK, Seoul, Korea) is a combination inhaler with a long-acting muscarinic antagonist (LAMA) plus a long-acting beta-2-agonist (LABA), licensed for COPD. Administered as a dry powder via oral inhalation [7], UMEC/VI has proven effective and safe for treating COPD and is approved in >100 countries, demonstrating superior lung function improvement over other LAMA/LABA fixed-dose combinations [8,9]. However, safety and efficacy data for UMEC/VI in Korean COPD patients are currently lacking.
Korean regulations mandate that new drugs should be re-examined and verified for adverse events (AEs) that did not appear during the approval process for 6 years after the initial approval date, and their safety and efficacy should be re-evaluated at 6, 12, 18, 24, 48, 60, and 72 months [10]. UMEC/VI was approved in Korea in 2014. In this study, we aimed to evaluate the safety and effectiveness of UMEC/VI in Korean patients using real-world data, according to post-marketing surveillance (PMS) regulations [10].
Materials and Methods
1. Study design
To demonstrate the safety of UMEC/VI (fixed-dose 62.5 μg/25 μg once daily) 6 years after its approval in Korea, this open-label, multicentre, observational, PMS study was conducted from July 10, 2014 to July 9, 2020, per re-examination regulations from the Korean Ministry of Food and Drug Safety [10].
Physicians conducted patient selection and enrolment processes. Data were collected by physicians from electronic clinical report forms (eCRFs) of patients consecutively diagnosed with COPD from Pulmonary or Pulmonary/Allergy Departments of 52 South Korean general hospitals. The follow-up period was 24 weeks after initial dose.
This study was conducted in accordance with the guidelines for Good Pharmaco-epidemiology Practices, and the ethical principles of the Declaration of Helsinki. The study protocol was approved by the Institutional Review Board of each study center including Kyungpook National University Hospital (IRB approval number: KNUMC PMS2015-027).
2. Patients
Eligible participants were those who received a COPD diagnosis during an outpatient visit, with a post-salbutamol forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) ratio <0.7, were ≥19 years of age, received UMEC/VI per domestically approved indications (fixed-dose 62.5 μg/25 μg once daily), and provided informed consent. Patients with asthma, acutely deteriorating COPD, hypersensitivity to UMEC/VI or its components, a severe milk protein allergy, galactose intolerance, Lapp lactase deficiency, or glucose-galactose malabsorption were excluded. There were no restrictions on concomitant drug usage.
3. Study endpoints
Safety endpoints were evaluated in patients who received ≥1 dose of UMEC/VI. Outcomes were based on the incidence of AEs, serious AEs (SAEs), adverse drug reactions (ADRs), and unexpected adverse events (UAEs). All AEs occurring in patients who completed the investigation were included in the safety analysis, irrespective of causality. Classification of AEs was based on the representative terms in Medical Dictionary for Regulatory Activities-Korea 22.1 (MedDRA-K 22.1).
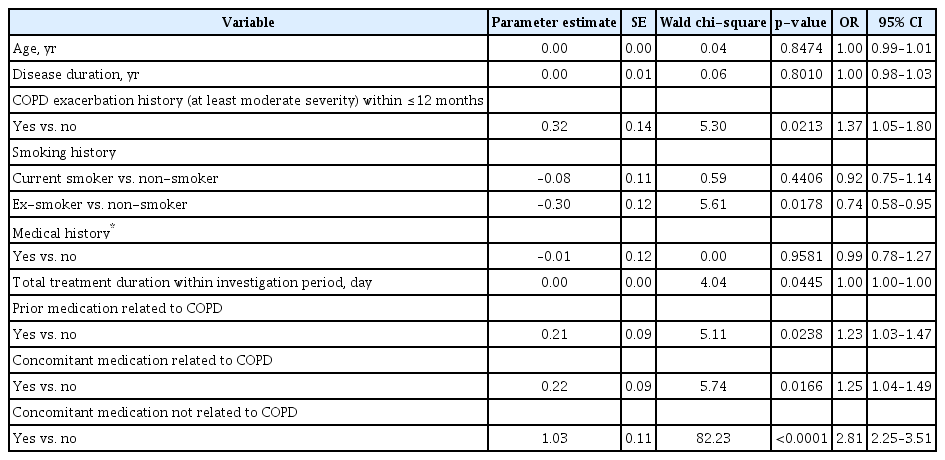
AEs were stratified by gender, age, body mass index (BMI), disease duration, COPD exacerbation of at least moderate severity within ≤12 months, smoking status, medical history, total treatment duration, prior medication related to COPD, concomitant medication related to COPD, and concomitant medication not related to COPD, and by special population, such as elderly patients (≥65 years of age) and patients with renal/hepatic impairment (defined per MedDRA‑K 22.1 terms). The total treatment period was defined as the period from the administration start date to the administration end date. If administration of the study drug was still being continued at the investigation end date, “administration end date” was replaced by “follow-up end date” in the analysis.
Effectiveness of UMEC/VI after 24 weeks of prescription initiation was assessed via physician’s evaluation of lung function improvement based on changes in post-bronchodilation (BD) FEV1 value according to the 2020 Global Initiative for Chronic Obstructive Lung Disease guidelines [11]. Individual physicians’ global assessments, rated ‘effective,’ ‘no change,’ ‘worsening,’ or ‘indeterminable’ according to local regulations were recorded, with ‘indeterminable’ cases excluded from further analysis. Lung function assessment was based on FEV1 change/mL 24 weeks after UMEC/VI prescription. Additional endpoints were change of BD FEV1% and improvement in the number and severity of exacerbations.
4. Statistical analyses
Statistical analyses were performed using SAS version software (SAS Institute Inc., Cary, NC, USA), with the crude number and rate of AE and ADR, described using mean and standard deviation (SD). For safety and effectiveness analyses, the percentage of patients reporting AEs were analysed using chi-square test or Fisher’s exact test, stratified by factors for subgroup analysis (95% confidence intervals and significance threshold of p<0.05) to identify variables independently affecting the incidence proportion among the special study group.
Multiple logistic regression analyses were performed using nine factors as independent variables (disease duration, COPD exacerbation of at least moderate severity, smoking status, medical history, total treatment duration, prior medication related to COPD, concomitant medication related to COPD, concomitant medication not related to COPD, and elderly population), and AE occurrence as a dependent variable. Continuous variables were used as independent variables for elderly population, disease duration, and total treatment duration.
5. Data availability
Information on GSK’s data sharing commitments and requesting access to anonymized individual participant data and associated documents can be found at www. clinicalstudydatarequest.com.
Results
1. Patient demographics
A total of 3,375 patients were identified for inclusion via retrieval of eCRFs in 52 hospitals, by 53 physicians; of these, 289 patients were excluded due to protocol violation. Thus, 3,086 patients were included in the safety analysis. Among those, 903 patients were included in the effectiveness analysis set (Figure 1).
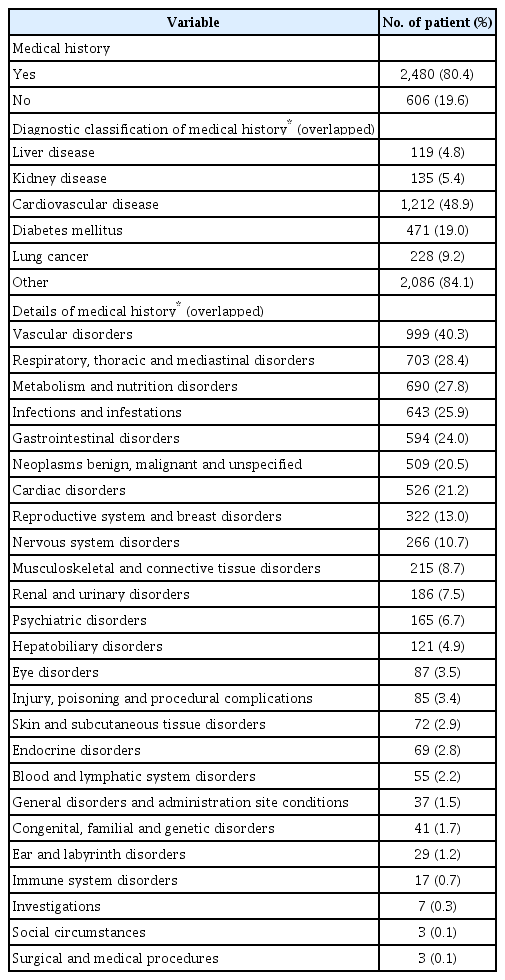
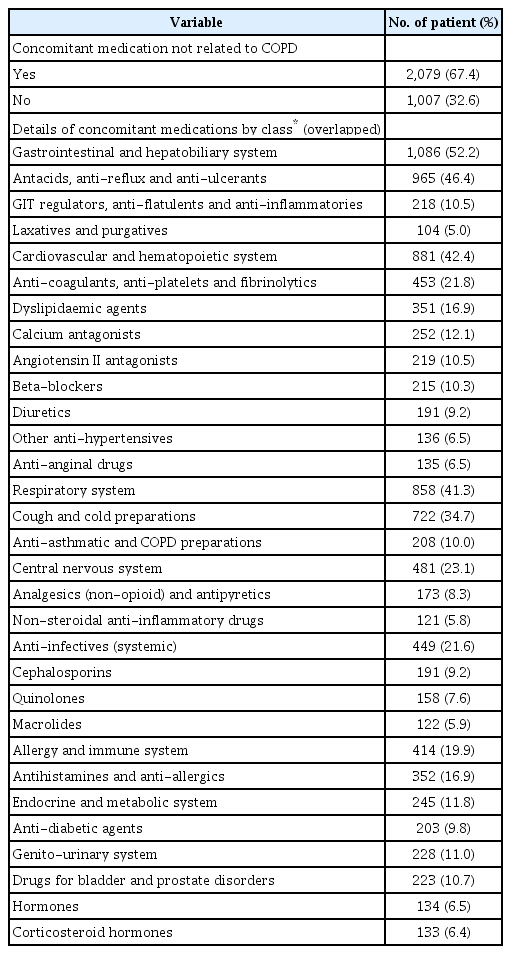
Of 3,086 patients, 85.9% were male and 73.1% were aged ≥65 years. Mean age was 69.8 years and mean duration of disease was 2.5 years. Ex-smokers comprised 52.6% of the population, current smokers 26.4%, and never smokers 21.0%. Overall, 9.0% of patients (n=279) experienced moderate or severe COPD exacerbation at least once within ≤12 months (Table 1) and 40.8% patients used COPD treatment prior to enrolment. Regarding prior medications (Table 1), 95.3% patients used a long-acting bronchodilator with or without an inhaled corticosteroid (ICS): ICS/LABA, n=450; LABA, n=200; LAMA, n=776; other LAMA/LABA, n=154 (except UMEC/VI). LAMA was the most frequently used treatment prior to using UMEC/VI (Table 1). In total, 80.4% of patients reported any medical history, except COPD (Table 2). Cardiovascular disease (CVD) accounted for the largest proportion of reported medical history (48.9%), followed by diabetes mellitus (19.0%) and lung cancer (9.2%) (Table 2). Of the 3,086 patients in the safety analysis set, 58.5% had concomitant medication related to COPD (Table 3). In terms of classification of medication, short-acting beta-2-agonists (SABA) accounted for the largest proportion (15.9%), followed by ICS/LABA (6.8%) and ICS alone (4.9%) (Table 3). Overall, 67.4% of patients had concomitant medication not related to COPD (Table 4). The highest proportion of these medications belonged to the gastrointestinal and hepatobiliary system class (52.2%), followed by the cardiovascular and hematopoietic system (42.4%) and the respiratory system (41.3%) (Table 4).
Patients were treated with UMEC/VI (74.2 μg/40 μg) for a mean 187 days. Overall, 898 patients successfully obtained a spirometry test; baseline mean±SD FEV1 and FVC were 1.55±0.55 and 2.95±0.84 L, respectively, and mean FEV1% predicted was 58.0%±16.3%.
2. Safety analysis
1) Incidence of AEs and ADRs
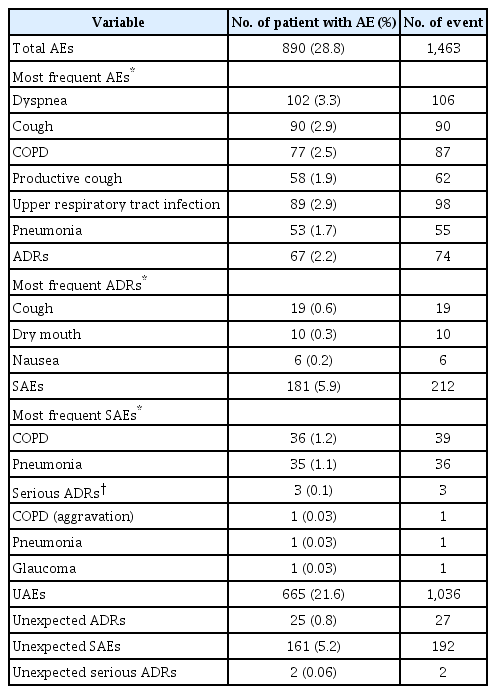
Overall, AEs were reported by 28.8% (n=890) of patients (Table 5). Respiratory, thoracic, and mediastinal disorders were the most reported AEs by system organ class (12.5%, n=385), followed by infections and infestations (6.7%, n=207), and gastrointestinal disorders (4.7%, n=146). In the analysis by preferred term, the most common AEs were dyspnea (3.3%, n=102), cough (2.9%, n=90), and upper respiratory tract infection (2.9%, n=89) (Table 5). Out of 890 patients with AEs, 2.2% (n=67) reported ADRs (Table 5), the most common being cough (0.6%, n=19), dry mouth (0.3%, n=10), and nausea (0.2%, n=6).
2) Incidence of SAEs and serious ADRs
SAEs were reported by 5.9% (n=181) of patients (Table 5); the most commonly reported SAEs were COPD, pneumonia, and dyspnea (Table 5). Serious ADRs were reported by three patients (0.1%; one aggravation of COPD, one pneumonia, one glaucoma) (Table 5). In the patient with COPD and the patient with pneumonia, symptoms resolved after appropriate treatment and UMEC/VI treatment was maintained; for the patient with glaucoma, UMEC/VI treatment was discontinued, and appropriate treatment given.
3) UAEs, severe UAEs with unexpected ADRs
UAEs were seen in 21.6% (n=665) of patients and unexpected ADRs in 0.8% (n=25) of patients (Table 5), with nausea being the most commonly reported (0.2%, n=6). Unexpected SAEs were reported by 5.2% (n=161) patients and two unexpected serious ADRs (0.01%) were reported (one COPD, one pneumonia).
4) Relevant factors with safety outcomes
Among 1,463 reported AEs, 1,071 were mild, 336 moderate, and 56 severe. A causal relationship with UMEC/VI was deemed ‘unlikely’ in 1,389 cases, ‘likely’ in 52 cases, ‘very likely’ in 18 cases, ‘certain’ in three cases, and ‘unevaluable’ in one case. Class-related AEs associated with high-dose LABA were rare (tremors [0.1%], blood pressure changes [0.1%], tachycardia, arrhythmia [<0.1%]), with no reporting of tachycardia or palpitations. Drug-related AEs associated with LAMAs, e.g., constipation (<1%), dryness of mouth (<1%), and urinary retention (<1%), occurred at low frequencies. There were 317 unresolved and 88 unknown cases, 16 deaths (deemed unlikely to be product-related by the investigator), and four sequelae cases. Treatment continued in 1,303 cases, while treatment was suspended permanently in 143 cases and temporarily in 12 cases; action taken was unknown in five cases, and appropriate versus inappropriate treatment was given in 939 versus 524 cases, respectively.
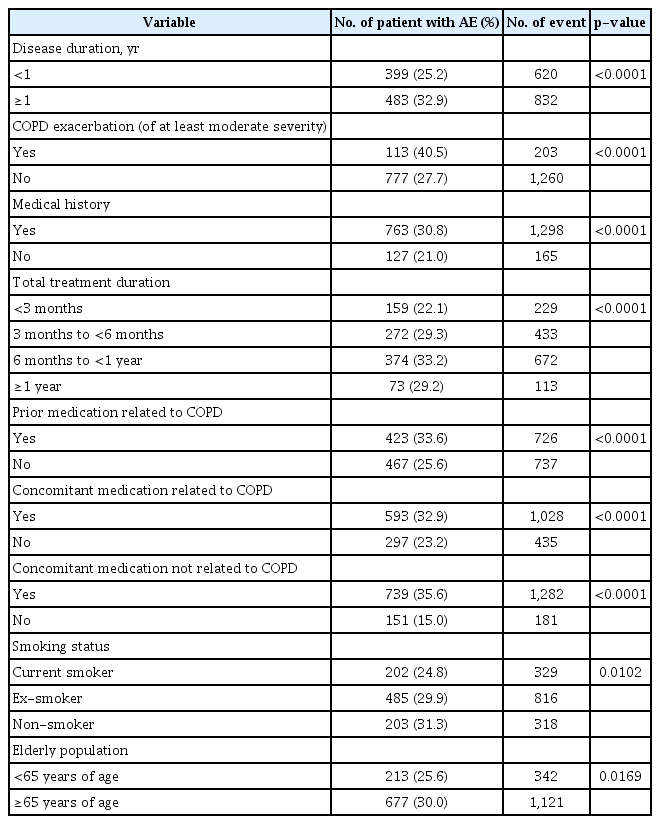
Differences in AE incidence were statistically significant for nine factors: disease duration, COPD exacerbation of at least moderate severity, medical history, total treatment duration, prior medication related to COPD, concomitant medication related to COPD, concomitant medication not related to COPD (all p<0.0001), smoking status (p=0.0102), and elderly population (p=0.0169) (Table 6).
Logistic regression was performed using the nine factors with statistically significant difference in AE incidence in the analysis by background factor as independent variables and AE occurrence as a dependent variable. As elderly population, disease period and total administration period were classified based on age, administration period and total administration period, respectively, continuous variables were used as independent variables in the analysis. As a result, it was found that the factors significantly affecting AE occurrence were COPD exacerbation at least moderate severity within recent 1 year (p=0.0213), smoking status (p=0.0178), total administration period (p=0.0445), prior medication related to COPD (p=0.0238), concomitant medication related to COPD (p=0.0166), and concomitant medication not related to COPD (p<0.0001) (Table 7).
No change in odds ratio was observed for increased treatment duration during the total treatment administration period. Patients who received the study drug for 6 months to <1 year reported the highest incidence of AEs at 33.2% (374/1127), followed by the 3 to <6 months group at 29.3% (272/929), the ≥1 year group at 29.2% (73/250), and the <3 months group at 22.1% (159/720) (p<0.0001 in univariate analysis) (Table 6). The odds ratio was 1.37 times higher in patients with COPD exacerbation of at least moderate severity within ≤12 months compared to those without, 1.23 times higher in patients with COPD-related prior medication compared to those without, 1.25 times higher in patients with COPD-related concomitant medication compared to those without, 2.81 times higher in patients with concomitant medication not related to COPD compared to those without, and 0.74 times lower in ex-smokers compared to non-smokers (Table 7).
3. Effectiveness analysis
Overall, 903 patients from the safety analysis set were included in the effectiveness analysis set (Figure 1). In total, 478 (52.9%) patients from the effectiveness analysis had received prior medication related to COPD, including long-acting bronchodilators with or without ICS. In addition, 548 (60.7%) of patients in the effectiveness analysis set reported concomitant medication related to COPD, including SABA, short-acting muscarinic antagonists (SAMA) and ICS with LABA. FEV1 values were collected before and after study drug administration in 898/903 (99.4%) patients in the effectiveness analysis. Mean FEV1±SD was 1.55±0.55 L before administration and 1.68±0.58 L after administration; mean change in FEV1 was 0.13±0.25 L (p<0.0001) (Figure 2A). Mean FVC was 2.95±0.84 L before administration and 3.06±0.85 L after administration; mean change in FVC was 0.12±0.38 L (p<0.0001) (Figure 2A). The mean FEV1/FVC ratio was 52.9%±11.8% before administration and 55.1%±12.5% after administration; mean change in FEV1/FVC ratio was 2.3%±6.1% (p<0.0001) (Figure 2B). Investigator-assessed effectiveness revealed 82.8% of patients improved (n=748), 15.7% were unchanged (n=142), and 1.4% had aggravated disease (n=13) (Figure 2C).
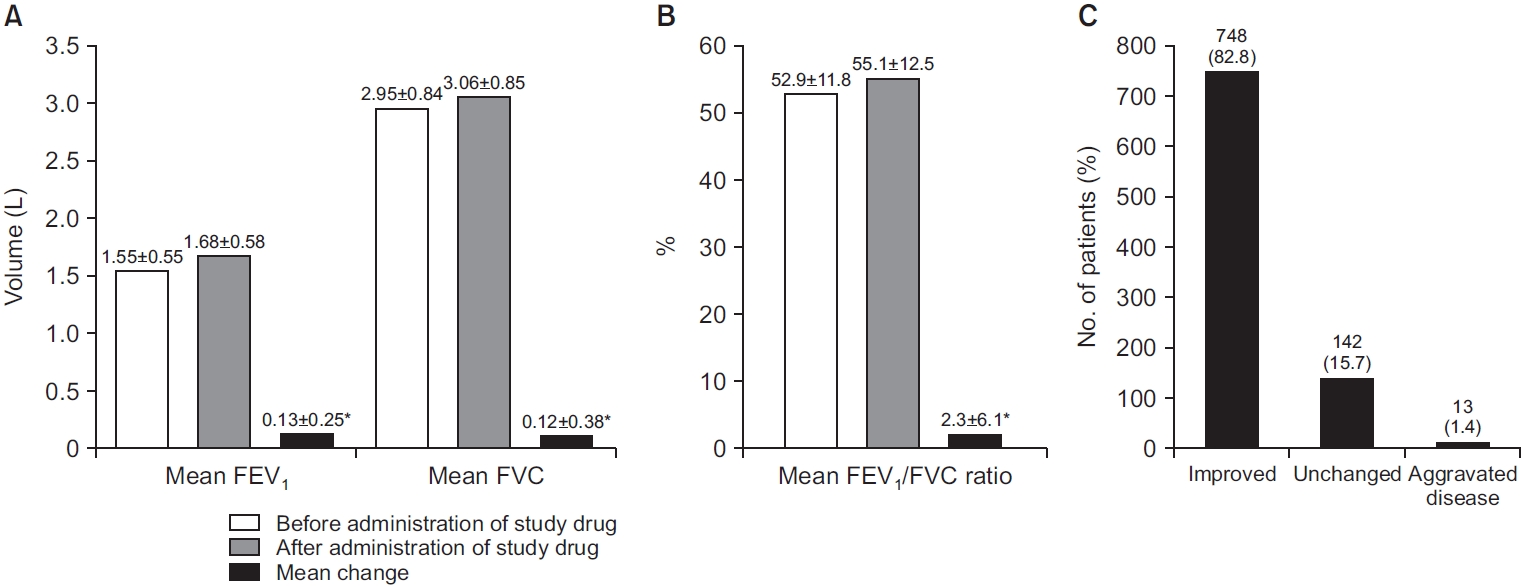
(A) Forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC) measurements of patients before/ after administration of study drug and mean change in FEV1. (B) Mean FEV1/FVC ratio measurements of patients before/after administration of study drug and mean change in FEV1/FVC ratio. (C) Investigator-assessed effectiveness. *p<0.0001.
Discussion
Patients with COPD in Korea display several unique features, including a high prevalence of tuberculosis, biomass smoke exposure and lower BMIs compared with Western populations [12,13]. AEs in COPD are known to increase in patients with low BMI or underlining diseases [14]. Therefore, it is important to verify the safety of UMEC/VI in Korean patients with multiple risk factors for COPD. This study on the safety of UMEC/VI in a high-risk population is important since there have been no safety studies of dual bronchodilators in Korea to date.
The population in this study was of a similar mean age, albeit different smoking history, to the general COPD population across South Korea [15]. A 2014 database review found the mean age of COPD patients across Korea was 69.3 years [16], compared with 69.8 years in our study. Furthermore, 2008 National Health and Nutrition Survey data established a sex disparity for COPD prevalence in Korea (men 19.4%; women 7.9%) [17]. In this study, 83.7% of patients were male and 80% of patients had underlying diseases (48.9% CVD, 19% diabetes, and 9.2% lung cancer), in line with other countries [18-20].
The incidence of AEs was 28.8%, and SAEs was 5.9%, lower than those reported in a previous phase 3 clinical trial (DB 2113373) [21], which reported on-treatment AEs in 51% (212/413) patients, and on-treatment SAEs in 5% of patients (21/413) [21]. The variation may be due to the different study designs and patient demographics between the studies. DB 2113373 was a 4-arm study with 24 weeks observation and more detailed inclusion/exclusion criteria than our study (e.g., symptomatic patients with a Modified Medical Research Council score >2 points were eligible for inclusion in DB 2113373; however, non-symptomatic patients were eligible for inclusion in our study because there were no inclusion criteria related to COPD symptoms).
Previously reported class effect AEs associated with high-dose LABA include tremors, blood pressure changes, tachycardia, arrhythmia, and palpitations [22,23]. These events were rare or not observed as were drug-related AEs associated with LAMAs; an unexpected small increase in cardiovascular events in COPD patients with ipratropium bromide, a SAMA, has been previously reported [24]. As both LAMA and LABA have possible class-related cardiac disorder effects, incidence of a rare drug-related AEs (palpitation, 0.1%) can be indicative of drug tolerability.
In this study, nine factors significantly affected the occurrence of AEs in univariate analyses: disease duration, COPD exacerbation of at least moderate severity within ≤12 months, smoking status, medical history, total treatment duration, prior COPD-related medication, COPD-related concomitant medication, concomitant medication not related to COPD, and older age. In a previous study, COPD exacerbation compromised health status, accelerated disease progression, and caused serious symptoms; function was irreversible to baseline levels in some patients and cardiovascular SAEs occurred more frequently [25]. This is in line with our findings that COPD exacerbation causes more frequent AEs.
In our study, never smokers were at a higher risk of developing an AE than former smokers. As smoking is reported as the greatest risk factor for COPD, and considering the COPD characteristics of irreversible airflow limitation are due to long-term exposure to risk factors [26], elucidation of the clinical meaning of these findings is difficult. AE incidence might have been affected by patient factors other than smoking history (e.g., COPD status at the time of surveillance participation, indirect smoke inhalation, etc.).
An association between AEs and prior medication related to COPD and concomitant medication both related and unrelated to COPD was observed in the present study. However, it is important to note that these medications may have caused the AE. As a result, it is difficult to consider the importance of these factors in a clinical setting despite their statistical significance. Patient medical history is another factor that significantly affected the occurrence of AEs in our study. There is evidence that CVD can affect COPD patients and is associated with various unfavorable outcomes, including reduced quality of life, increased hospitalization risk, and increased risk for all-cause and CVD mortality [27]. Almost half the patients in our study with a medical history, other than COPD, reported CVD. Therefore, this may account for the association between medical history and occurrence of AEs observed in our study.
Increased risk of cardiovascular events, leading to hospitalization and emergency department visits, has previously been reported in new users or bronchodilators [28]. One study found that cardiovascular events in new users were highest in the first 3 weeks after the medication was prescribed [28]. The findings from our study showed that treatment duration significantly affected the incidence of AEs. This association may have been due to an increased incidence of AEs in new users in our study; however, this was not formally assessed.
Patient age was another factor identified in our study, with older age significantly affecting the occurrence of AEs. A long-term safety study of tiotropium/olodaterol in patients with COPD reported that the proportion of patients that reported AEs was higher in older patients [29]. The increased risk of AEs in older patients with COPD may be due to an increase in comorbidities and concomitant medications in this patient group [29,30]. This is consistent with our findings that showed that older age, concomitant medication and medical history all significantly affected the occurrence of AEs.
In this study, UMEC/VI was shown to be effective in 82.8% of patients, with a mean±SD change in FEV1 of 0.13±0.25 L. This improvement was not as marked as data from a global phase 3 efficacy study of UMEC/VI, DB 2113373 [21]; however, baseline lung function was higher in our study which may account for the differences in lung function improvements observed between the two studies.
In terms of limitations, our study was an open-label study without a control group, therefor it was only possible to measure the improvement of lung function before and after UMEC/VI use. In addition, since this is an observational study, AEs were collected by patients’ self-reported data. In this open-label, observational, multicentre, PMS study, the licensed dose of UMEC/VI trifenatate (62.5 μg/25 μg) was well-tolerated over 24 weeks of treatment in patients with COPD in Korea. AEs thought to be a ‘class effect’ of LAMA/LABA treatment were either not or rarely observed during the course of this 6-year post-marketing period. Five factors significantly affected the odds of an AE in multivariate analyses (COPD exacerbation severity, COPD-related prior and concomitant medications, non-COPD-related concomitant medication, and smoking). Of the 903/3,086 patients analysed for effectiveness, the majority (82.8%) showed overall disease improvement after UMEC/VI treatment. The results of this study further support the safety and effectiveness of UMEC/VI for the treatment of COPD.
Notes
Authors’ Contributions
Conceptualization: Cho JE. Formal analysis: Cho EY, Cho JE, Lee EB. Investigation: Yoo SS, Chang JH. Writing - original draft preparation: Cho EY. Writing - review and editing: Cho EY, Cho JE, Lee EB, Yoo SS, Chang JH. Approval of final manuscript: all authors.
Conflicts of Interest
Eun-Yeong Cho was an employee and shareholder, Jung-Eun Cho, and Eun-Bin Lee are employees of GSK. Seung Soo Yoo and Jung Hyun Chang disclosed no conflict of interests.
Funding
This study (204511) was funded by GSK. Trademarks are owned by or licensed to their respective owners (GSK [ANORO ELLIPTA]).
Acknowledgements
Editorial support for the development of this manuscript, under the direction of the authors, was provided by Rebecca Benatan, BSc, and Fiona Scott, Ph.D., of Ashfield MedComms, Macclesfield and Glasgow, UK, an Ashfield Health Company, and was funded by GSK.