Serial Changes in Mannose-Binding Lectin in Patients with Sepsis
Article information
Abstract
Background
Mannose-binding lectin (MBL) deficiency leads to increased susceptibility to infection. We investigated whether serial changes in MBL levels are associated with the prognosis of patients diagnosed with septic shock, and correlated with cytokine levels.
Methods
We enrolled 131 patients with septic shock in the study. We analyzed the serum samples for MBL and cytokine levels at baseline and 7 days later. Samples on day 7 were available in 73 patients.
Results
We divided the patients with septic shock into four groups according to serum MBL levels (<1.3 µg/mL or ≥1.3 µg/mL) on days 1 and 7. Patients with low MBL levels on day 1 and high MBL levels on day 7 showed a favorable prognosis for 28-day survival (odds ratio, 1.96, 95% confidence interval, 1.10–2.87; p=0.087). The high MBL group on day 7 showed a significant decrease in monocyte chemoattractant protein 1, interleukin (IL)-1β, IL-6, IL-8, interferon-γ, and granulocyte macrophage colony-stimulating factor levels compared with the low MBL group on day 7.
Conclusion
The increase in MBL levels of patients with septic shock may suggest a favorable prognosis and attenuate pro-inflammatory and anti-inflammatory responses.
Introduction
Septic shock remains an important cause of mortality and morbidity in intensive care units (ICUs)123. Numerous studies suggest that individuals vary in their ability to resist infections456 and that this susceptibility to the infection is associated with lethal complications unrelated to the cause of illness. Therefore, it is important to identify accurate predictors of the prognosis of patients with septic shock. The innate immune system is activated prior to the adaptive immune system, and is thus the first line of defense against pathogens. The importance of the interactions between pathogen-associated microbial patterns and mannose-binding lectin (MBL) in activating innate immunity is well established as a component of the innate immune system7. In addition, it is now recognized that the first response to invasion (i.e., innate immunity) has a significant influence on the subsequent adaptive response89.
It has been reported that individuals with a deficiency in MBL, a key recognition molecule in the complement lectin pathway, are susceptible to infections101112. Moreover, low levels of MBL cause defects in opsonization and phagocytosis that have been associated with recurrent infections in infants and adults111314. Cytokines are secreted by components of the innate and adaptive immune systems and act as modulators of inflammatory responses15. MBL determines which cytokines are released because of its interaction with phagocytic cells16.
We hypothesized that patients who have a low response in increasing MBL levels after infection may be at high risk of death. To address this question, we investigated whether serial changes in MBL levels are associated with the prognosis of septic shock. We also assessed whether the serial changes of MBL affected cytokine levels.
Materials and methods
1. Study subjects
In this study, 131 patients receiving intensive care for septic shock were enrolled. Follow-up samples on day 7 were obtained in 73 patients. All patients were older than 18 years and had been admitted to the ICU of a tertiary teaching hospital in Seoul, South Korea. The diagnosis of septic shock was based on the criteria presented at the American Society of Chest Physicians/Society of Critical Care Medicine Consensus Conference in 1992: evidence for infection, evidence of a systemic response to infection, systolic blood pressure of less than 90 mm Hg despite adequate fluid resuscitation, and hypoperfusion or organ dysfunction attributable to sepsis17. In a previous study, we reported that a low MBL level (<1.3 µg/mL) is an independent risk factor for mortality after 28 days in patients with septic shock18. Patients were categorized based on their MBL levels (<1.3 µg/mL or ≥1.3 µg/mL) on days 1 and 7. Informed consent was obtained from all study participants or their relatives in accordance with the policies of the institutional review board. This study was approved by the institutional review board (IRB No. 2005-0070).
Clinical data including demographic details, the sequential organ failure assessment (SOFA) score, the acute physiology, age, and chronic health evaluation II (APACHE II) score obtained on admission, and the ICU outcome, were recorded for each patient.
2. Quantification of MBL
Blood samples were collected within 24 hours after the onset of septic shock and on week 1 if the patients were alive in the ICU. The serum MBL level was measured using a sandwich enzyme-linked immunosorbent assay (MBL-ELISA; Dobeel Co., Seongnam, Korea) according to a previously established protocol1819.
3. Cytokine measurement
The serum levels of the proinflammatory cytokines interleukin (IL)-1β, IL-6, IL-8, monocyte chemoattractant protein 1 (MCP-1), interferon-γ (IFN-γ), tumor necrosis factor α (TNF-α), and the anti-inflammatory cytokines IL-1 receptor antagonist (IL-1ra), IL-10, and granulocyte-macrophage stimulating factor (GM-CSF). were measured by using a multiplex bead-based assay (Bio-Rad, Hercules, CA, USA).
4. Statistical analysis
The values obtained for the continuous variables were not normally distributed; therefore, the results were expressed as medians with an interquartile range. All categorical data were compared using chi-square analysis or Fisher exact test. The Mann–Whitney test was used to test for differences between the two groups. Associations between cytokines were measured with Spearman's rank correlation. The prognostic value of the MBL level was calculated using bootstrapping, a nonparametric method that takes 1,000 samples of the data. All data were analyzed using SPSS version 20.0 software (IBM Corp., Armonk, NY, USA).
Results
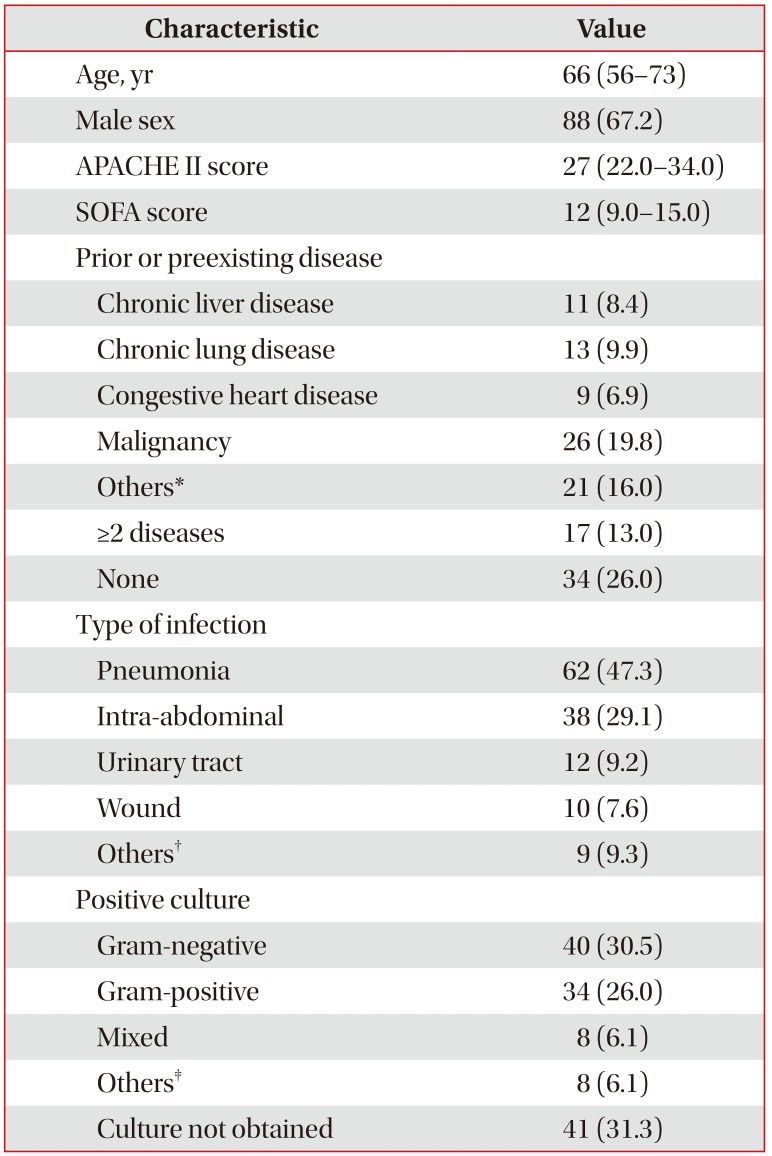
Baseline demographic, clinical, and microbiological data of all included patients (n=131) are summarized in Table 1. The median age was 66 years. Eighty-eight men (67.2%) and 43 women (32.8%) were included. The most common cause of septic shock was pneumonia. The 28-day overall mortality rate was 35.9%.
Because we measured serum MBL levels on days 1 and 7 in 73 patients with septic shock, only 73 patients were enrolled in the final analysis. The median serum MBL level was 1.75 mg/mL (range, 0.84–2.65 µg/mL) on day 1 and 2.17 µg/mL (range, 1.0–3.66 µg/mL) on day 7. The serum MBL level on day 1 was negatively correlated with the SOFA score (r=−0.237, p=0.015).
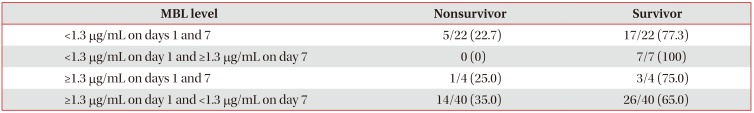
We divided the patients with septic shock based on their serum MBL levels (<1.3 µg/mL or ≥1.3 µg/mL) on days 1 and 7. The group with a low MBL level (<1.3 µg/mL) on day 1 and increased MBL level (≥1.3 µg/mL) on day 7 had a good prognosis for 28-day survival (odds ratio, 1.96; 95% confidence interval, 1.10–2.87; p=0.087) (Table 2).
The group with low MBL levels showed a significant increase in MCP-1, IL-6, IL-8, IFN-γ, and IL-1ra levels compared with the group with high MBL levels (≥1.3 µg/mL) on day 1 (Table 3). Though the levels of the inflammatory and anti-inflammatory cytokines on day 7 was decreased, the group with low MBL (< 1.3 µg/mL) showed a significant increase in IL-8 level compared with the group with higher MBL (Table 4).
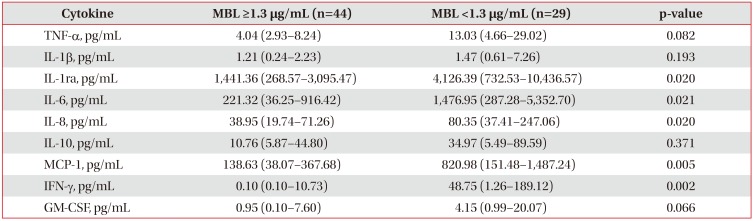
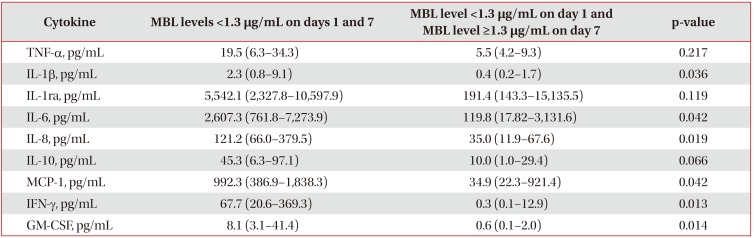
Even though the initial MBL level was low, the group with increased MBL levels on day 7 showed a significant decrease in MCP-1, IL-1β, IL-6, IL-8, IFN-γ, and granulocyte macrophage colony-stimulating factor levels (Table 5).
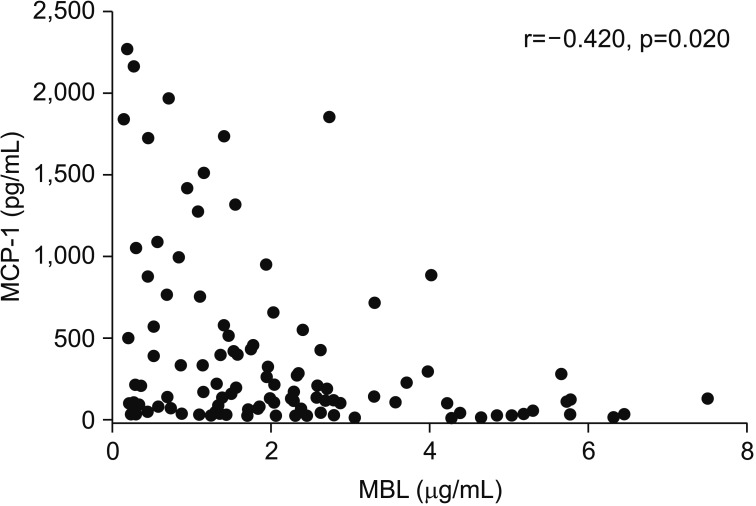
The levels of MCP-1 (r=−0.420, p=0.020) were negatively correlated with the MBL level on day 1 (Figure 1).
Discussion
We reported in a previous study that the initial MBL level (≥1.3 µg/mL) is an independent predictive factor of survival in septic shock18. In this study, we observed that the increase of serum MBL level (≥ 1.3 µg/mL) on day 7 was a good prognostic factor, even though initial MBL levels were low (<1.3 µg/mL) in patients with septic shock.
Furthermore, to investigate the relationship of MBL levels with key pro-/anti-inflammatory cytokines in sepsis, we compared the cytokine profile and MBL levels in patients with septic shock. The group with high MBL levels showed significantly lower levels of pro- and anti-inflammatory cytokines such as IL-1ra, IL-6, IL-8, MCP-1, and IFN-γ compared to the group with low MBL levels (<1.3 µg/mL) on day 1. Complement activation through MBL might contribute to pro- and anti-inflammatory responses in the early stage of sepsis. In addition, if the immune response of pro-inflammatory cytokines, such as IL-1β, IL-6, IL-8, MCP-1, GM-CSF, and IFN-γ at initial was attenuated, MBL levels on day 7 could be restored. This subgroup showed good prognosis, even though the MBL level on day 1 was low.
Low levels of MBL result in defects in opsonization and phagocytosis1113. Although the relationship between cytokines and MBL is not completely clear, MBL, a component of the innate immune system, downregulates monocyte-mediated inflammation while enhancing phagocyte recruitment, thereby influencing the adaptive immune response10202122. Inflammatory monocytes respond rapidly to microbial stimuli by secreting cytokines and antimicrobial factors; for example, they express the chemokine receptor CCR2 and traffic to sites of microbial infection in response to MCP-1 secretion23. MCP-1 is a potent chemoattractant of mononuclear cells and an important immunomodulator in control of the balance between pro- and anti-inflammatory responses in sepsis24. Although we did not investigate monocyte differentiation and function, our study shows a significant negative correlation between MBL and MCP-1 in patients with septic shock. Moreover, IL-8 production is induced by bacteria, viruses, and other proinflammatory cytokines (IL-1β and TNF-α) and IL-8 is identified as a chemotactic factor for neutrophils25. Activation of the complement system promotes the opsonization and killing of bacteria as well as the inflammatory response. In our study, these effects of MBL might suppress IL-8 level on days 1 and 7.
This study has limitations by the small sample size and the selection bias because we could not analyze all samples of the enrolled patients on day 7. Only patients who survived after 7 days were included to compare MBL levels on day 1 with day 7. We could not provide strong evidence supporting the relationship between MBL levels and cytokine profile in vivo and in vitro, and we compared the results of the MBL levels and cytokine levels using the cut-off level of our previous study because reference value has not been established.
In conclusion, our results indicate that the serial change in MBL levels may associate with the outcome of septic patients. Moreover, we observed the possibility of a role of MBL function in immune modulation. This result needs to be confirmed in a large cohort study and in vitro studies.
Notes
Authors' Contributions: Conceptualization: Huh JW, Koh Y. Acquisition of data: Huh JW, Hong SB, Lim CM. Analysis of data: Song K, Yum JS. Interpretation of data: Huh JW, Kim HJ. Writing - original draft preparation: Huh JW, Koh Y. Writing - review and editing: all authors. Approval of final manuscript: all authors.
Conflicts of Interest: No potential conflict of interest relevant to this article was reported.