Factors Associated with the Discrepancy between Exercise Capacity and Airflow Limitation in Patients with Chronic Obstructive Pulmonary Disease
Article information
Abstract
Background
Exercise capacity is associated with lung function decline in chronicobstructive pulmonary disease (COPD) patients, but a discrepancy between exercisecapacity and airflow limitation exists. This study aimed to explore factors contributingto this discrepancy in COPD patients.
Methods
Data for this prospective study were obtained from the Korean COPD SubgroupStudy. The exercise capacity and airflow limitation were assessed using the6-minute walk distance (6-MWD; m) and forced expiratory volume in 1 second (FEV1).Participants were divided into four groups: FEV1 >50%+6-MWD >350, FEV1 >50%+6-MWD ≤350, FEV1 ≤50%+6-MWD >350, and FEV1 ≤50%+6-MWD ≤350 and their clinicalcharacteristics were compared.
Results
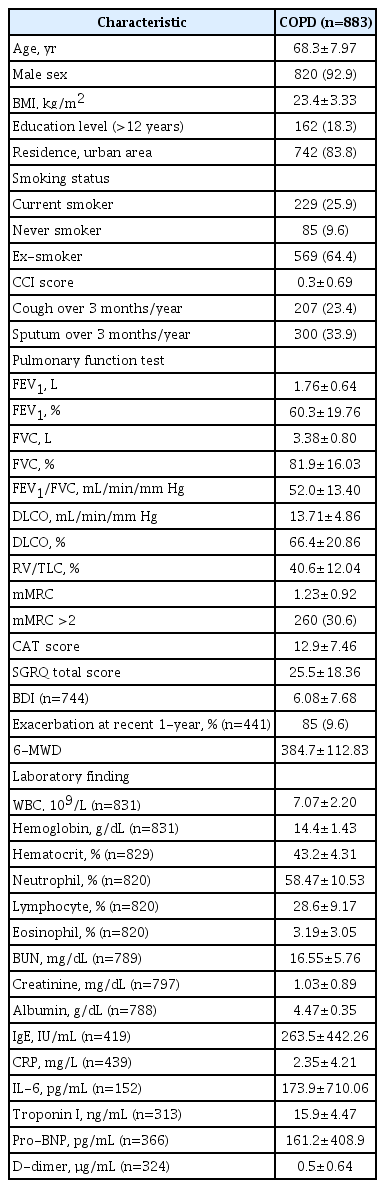
A total of 883 patients (male:female, 822:61; mean age, 68.3±7.97 years) wereenrolled. Among 591 patients with FEV1 >50%, 242 were in the 6-MWD ≤350 group, andamong 292 patients with FEV1 ≤50%, 185 were in the 6-MWD >350 group. The multipleregression analyses revealed that male sex (odds ratio [OR], 8.779; 95% confidence interval[CI], 1.539 to 50.087; p=0.014), current smoking status (OR, 0.355; 95% CI, 0.178to 0.709; p=0.003), and hemoglobin levels (OR, 1.332; 95% CI, 1.077 to 1.648; p=0.008)were significantly associated with discrepancies in exercise capacity and airflow limitationin patients with FEV1 >50%. Meanwhile, in patients with FEV1 ≤50%, diffusioncapacity of carbon monoxide (OR, 0.945; 95% CI, 0.912 to 0.979; p=0.002) was significantlyassociated with discrepancies between exercise capacity and airflow limitation.
Conclusion
The exercise capacity of COPD patients may be influenced by factors otherthan airflow limitation, so these aspects should be considered when assessing andtreating patients.
Introduction
Reduced exercise capacity is a key clinical feature in chronic obstructive pulmonary disease (COPD) patients [1] who are usually associated with a decline in lung function, particularly forced expiratory volume in 1 second (FEV1) [2].
Studies on the association between the degree of airflow limitation and exercise capacity in COPD revealed that patients with minor airflow limitation do not have a decreased exercise capacity. However, patients with moderate airflow limitation are reported to exhibit a reduced exercise capacity [3]. In a study comprising 118 COPD patients, the 6-minute walk distance (6-MWD) test results, used to assess exercise capacity, indicated that the performance of patients with severe airflow limitation was approximately 30% worse than those with mild airflow limitation [4].
However, the severity of the airflow limitation in COPD patients cannot be used to accurately predict a decline in exercise capacity. Spruit et al. [5]’s study including 1,795 individuals with COPD investigated the predictors of poor 6-MWD test results (≤350 m). They reported that, in addition to significant airflow limitation, a high degree of emphysema, more depressed mood, and greater perception of dyspnea symptoms were related to a decreased exercise capacity [5]. Furthermore, regardless of the airflow limitation level, patients more vulnerable to dynamic hyperinflation display a more reduced exercise capacity [6,7].
These studies suggest that decreased exercise capacity in COPD is highly complex, involving a wide range of contributing factors. In clinical practice, we often encounter patients with preserved exercise capacity despite significant airflow limitations, while others exhibit profoundly reduced exercise capacity despite mild airflow restrictions. Thus, this study aimed to identify the variables that contribute to the discrepancy between exercise capacity and airflow limitation in COPD patients.
Materials and Methods
1. Study population
This study included patients from the Korean COPD Subgroup Study cohort, since December 2011, a multicenter, prospective observational cohort of COPD patients from 53 centers in South Korea. The inclusion criteria were over 40 years old and a postbronchodilator FEV1/forced vital capacity (FVC) ratio of 0.7. To enroll the patients, physicians or skilled nurses used case-report forms to record information such as age, sex, height, weight, smoking status, patient-reported education level, area of residence, comorbidities, and respiratory symptoms such as cough and sputum production. In addition, results for 6-MWD, the COPD assessment test (CAT), COPD-specific St. George’s Respiratory Questionnaire (SGRQ), Beck Depression Inventory (BDI) score, and modified Medical Research Council (mMRC) score for dyspnea severity were evaluated [8]. A serologic laboratory test was also performed. During the first year of follow-up after enrollment, the occurrence and frequency of exacerbations were examined.
2. Pulmonary function test, 6-MWD test, and COPD severity
During enrolment, spirometer, diffusion capacity of carbon monoxide (DLCO), lung volume measurement, and 6-MWD were measured according to the recommendations of the American Thoracic Society and European Respiratory Society [9]. The severity of COPD was classified according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guideline. Lung function severity was classified as GOLD stage 1 (FEV1 ≥80%), GOLD stage 2 (50%≤ FEV1 <80%), GOLD stage 3 (30%≤ FEV1 <50%), and GOLD stage 4 (FEV1 <30%).
3. Group assignment
The patients were divided into four groups according to their FEV1 and 6-MWD results. To differentiate between mild to moderate and severe to very severe airflow limitation, the threshold for FEV1 was 50%, which separates GOLD stages 1–2 from GOLD stages 3–4. To distinguish between high and low exercise capacity among the participants, the threshold for the 6-MWD was set as 350 m, which was considered “poor 6-MWD” in the Evaluation of COPD Longitudinally to Identify Predictive Surrogate End-points (ECLIPSE) study [10]. Therefore, the four groups were as follows: group 1 (high FEV1 >50% and high 6-MWD >350 m), group 2 (high FEV1 >50% and low 6-MWD ≤350 m), group 3 (low FEV1 ≤50% and high 6-MWD >350 m), and group 4 (low FEV1 ≤50% and low 6-MWD ≤350 m).
4. Ethics statement
This study adhered to the Declaration of Helsinki guidelines, and written informed consent was obtained from all the participants. The study was approved by the Institutional Review Board of Gyeongsang National University Changwon Hospital (GNUCH-2023-03-029).
5. Statistical analyses
Categorical variables are reported as frequencies and percentages, whereas all continuous variables are described as mean and standard deviation. To examine continuous variables for normal and nonnormal data distributions, respectively, a two-sample t-test or the Mann-Whitney U test was applied and the Fisher’s exact test or the chi-squared test was used to assess categorical values. Multiple logistic regression analyses were conducted to assess the components that may contribute to the discrepancy between exercise capacity and airflow limitation. Covariates were selected for the logistic regression model based on univariate analysis (p>0.2) and clinical relevance. Age, sex, body mass index (BMI), smoking status, the Charlson comorbidity index (CCI), lung volume, the DLCO, and FEV1 values from pulmonary function tests, the CAT and BDI scores, and the mMRC score were covariates along with exacerbation from the prior year and laboratory test. A p-value of 0.05 was regarded as statistically significant for all comparisons. The SPSS version 25.0 for Windows (IBM Company, Armonk, NY, USA) was used for all statistical analyses.
Results
1. Baseline characteristics
A total of 883 patients were included in the study. We only included patients who had completed the 6-MWD test and pulmonary function tests, including diffusion capacity and lung volume measurements. These patients completed the CAT and SGRQ (Figure 1). The median age of the 883 patients was 70 years (interquartile range, 64 to 74), with males accounting for 92.9% of the study population. The mean BMI of the patients was 23.4±3.33 kg/m2, and current smokers accounted for 64.3% of the study population. The CCI was 0.3±0.69. The FEV1 was 1.76±0.64 and %FEV1 was 60.3±19.76. The DLCO (%) was 66.4±20.86 and residual volume (RV)/total lung capacity (TLC) (%) was 40.6±12.04. The 6-MWD was 384.7±112.83 m. CAT and SGRQ total scores were 12.9±7.46 and 25.5±18.36, respectively. Table 1 shows the baseline characteristics of the study population.
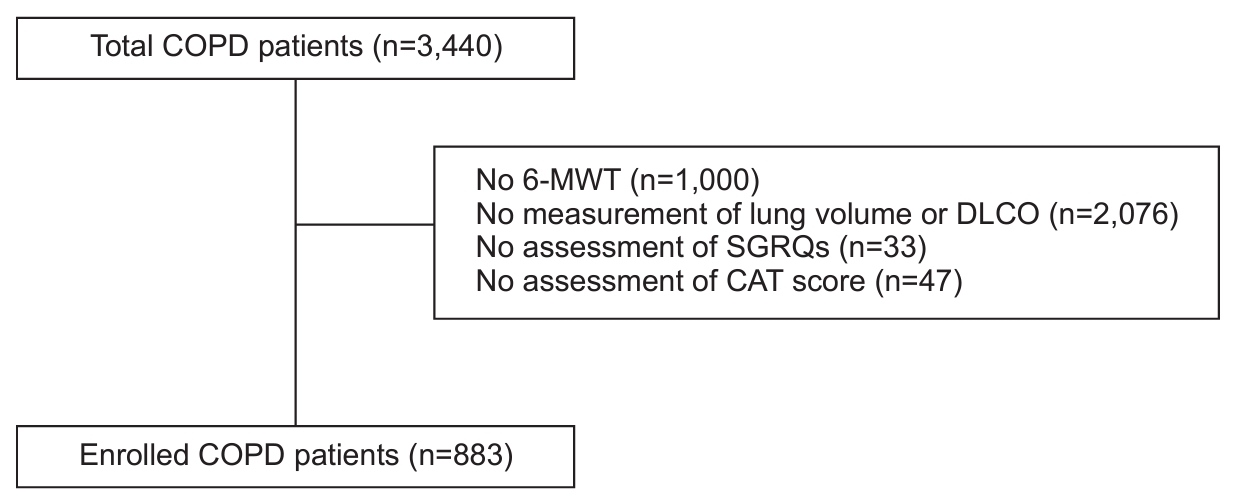
Flow diagram of the study population. COPD: chronic obstructive pulmonary disease; 6-MWD: 6-minute walk distance; DLCO: diffusion capacity carbon monoxide; SGRQ: St. George’s Respiratory Questionnaire; CAT: COPD assessment test.
2. Comparison of the clinical characteristics of four groups of COPD patients
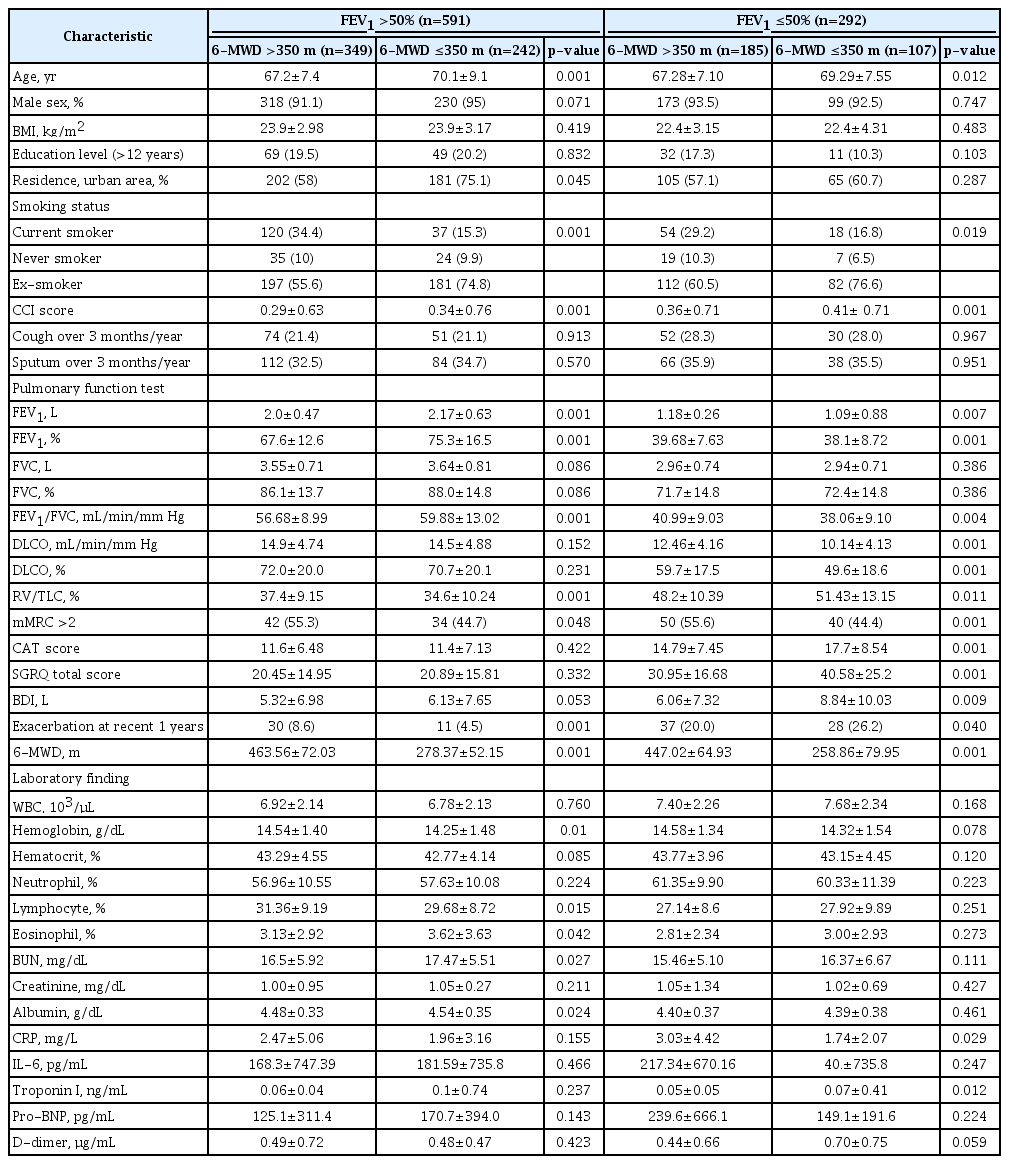
The characteristics of the groups, including pulmonary function and 6-MWD, are shown in Table 2. There were 591 patients with FEV1 >50% and 292 patients with FEV1 ≤50%. Among the 591 patients with FEV1 >50%, 349 (59%) and 242 (41%) patients had 6-MWD >350 m and ≤350 m, respectively (Figure 2). Age and the CCI were substantially higher in the 6-MWD ≤350 m group than in the 6-MWD >350 m group (70.1±9.1 vs. 67.2±7.4, p=0.001; 0.34±0.76 vs. 0.29±0.63, p=0.001; respectively). However, the proportion of current smokers was significantly higher in the 6-MWD >350 m group than in the 6-MWD ≤350 m group (34.4 vs. 15.3, p=0.001). For the pulmonary function test, the FEV1 (L), FVC (%), and FEV1/FVC (%) were significantly higher in the 6-MWD ≤350 m group than in the 6-MWD >350 m group (2.0±0.47 vs. 2.17±0.63, p=0.001; 67.6±12.6 vs. 75.3±16.5, p=0.001; 56.68±8.99 vs. 59.88±13.02, p=0.001; respectively). RV/TLC (%) was significantly higher in the 6-MWD >350 m group than in the 6-MWD ≤350 m group (37.4±9.15 vs. 34.6±10.24, p=0.001). The 6-MWD was significantly higher in the 6-MWD >350 m group than in the 6-MWD ≤350 m group (463.56±72.03 vs. 278.37±52.15, p=0.001). In addition, the hemoglobin levels and lymphocyte count (%) were significantly lower in the 6-MWD ≤350 m group than in the 6-MWD >350 m group (14.25±1.48 vs. 14.54±1.40, p=0.01; 29.68±8.72 vs. 31.36±9.19, p=0.015; respectively).

Classification of the patients into four groups according to forced expiratory volume in 1 second (FEV1) and 6-minute walk distance (6-MWD). COPD: chronic obstructive pulmonary disease.
In 292 patients with FEV1 ≤50%, 185 (63.3%) and 107 (36.7%) patients were in the 6-MWD >350 m and ≤350 m groups, respectively. Age and the CCI were significantly higher in the 6-MWD ≤350 m group than in the 6-MWD >350 m group (69.29±7.55 vs. 67.28±7.1, p=0.012; 0.41±0.71 vs. 0.36±0.71, p=0.001; respectively). However, the proportion of current smokers was significantly higher in the 6-MWD >350 m group than in the 6-MWD ≤350 m group (29.2 vs. 16.8, p=0.019). The pulmonary function test revealed that the FEV1 (L), FEV1/FVC (%), and DLCO (%) were significantly lower in the 6-MWD ≤350 m group than in the 6-MWD >350 m group (1.09±0.88 vs. 1.18±0.26, p=0.007; 38.06±9.10 vs. 40.99±9.03, p=0.004; 49.6±18.6 vs. 59.7±17.5, p=0.001; respectively). RV/TLC (%) was significantly higher in the 6-MWD ≤350 m group than in the 6-MWD >350 m group (51.43±13.15 vs. 48.2±10.39, p=0.001). For the laboratory findings, the troponin I level was significantly higher in the 6-MWD ≤350 m group than in the 6-MWD >350 m group (0.07±0.41 vs. 0.05±0.05, p=0.012). CAT score, SGRQ total score, and BDI were significantly higher in the 6-MWD ≤350 m group than in the 6-MWD >350 m group (17.7±8.54 vs. 14.79±7.45, p=0.001; 40.58±25.2 vs. 30.95±16.68, p=0.001; 8.84±10.03 vs. 6.06±7.32, p=0.009; respectively).
3. Factors associated with the discrepancy between airflow limitation and exercise capacity in mild to moderate COPD patients
Multiple logistic regression analyses revealed that males (odds ratio [OR], 8.779; 95% confidence interval [CI], 1.539 to 50.087; p=0.014), current smoking status (OR, 0.355; 95% CI, 0.178 to 0.709; p=0.003), and hemoglobin levels (OR, 1.332; 95% CI, 1.077 to 1.648; p=0.008) were substantially related to differences in exercise capacity and airflow limitation in patients with mild to severe COPD (Table 3). This discrepancy was not linked to pulmonary function test results or laboratory findings.
4. Factors associated with the discrepancy between airflow limitation and exercise capacity in severe to very severe COPD patients
Multiple logistic regression analyses showed that DLCO (OR, 0.945; 95% CI, 0.912 to 0.979; p=0.002) was substantially associated with the discrepancy between airflow limitation and exercise capacity in patients with severe to very severe COPD (Table 4). There was no correlation between the severity of airway obstruction, smoking status, and laboratory results.
Discussion
This study investigated the characteristics and factors that are related to reduced exercise capacity despite mild to moderate airflow limitation and a higher exercise capacity despite severe to very severe airflow limitation in patients who had COPD. The main findings of this study were as follows. First, many COPD patients showed a discrepancy between the degree of airflow limitation and exercise capacity. In this study, 40% of the COPD patients with mild to moderate airflow limitation (FEV1 >50%) exhibited a lower 6-MWD (≤350). Conversely, 36% of COPD patients with severe to very severe airflow limitation (FEV1 ≤50%) exhibited a higher 6-MWD (>350). Second, in patients with mild to moderate airflow limitation, male sex, smoking status, and hemoglobin level were associated with a discrepancy between airflow limitation and exercise capacity. In COPD patients with severe to very severe airflow limitation, the diffusion capacity of the lung was found to have an association with this discrepancy.
In this study, smoking status was associated with discrepancies between the degree of airflow limitation and exercise capacity in COPD patients with mild and moderate airflow limitation. It was found that patients with mild to moderate COPD who were current smokers had a considerably better capacity for exercise than anticipated. A previous study in China that compared the clinical characteristics of 4,331 COPD patients between current and former smokers indicated that current smokers have higher exercise capacity than former smokers [11]. This data reflects that in situations when the degree of airway obstruction is less severe, those with substantially higher exercise capacity are more likely to continue smoking. However, several studies have demonstrated that smoking status may have a negative influence on exercise capacity in healthy older individuals and patients with COPD. In another study of 154 older healthy individuals, 6-MWD according to their smoking status was measured [12]. Although the level of physical activity did not differ according to smoking status, current and ex-smokers had about 20% shorter 6-MWD than nonsmokers. In addition, current smokers without COPD showed a shorter 6-MWD than nonsmokers. Although handgrip and limb muscle strength were not different, the average 6-MWD in nonsmokers was significantly higher than that in smokers [13]. In addition, COPD patients who stopped smoking showed shorter 6-MWD than those who did not stop (305.23±58.36 m vs. 368.87±53.49 m, respectively), but had similar airflow obstruction (1.44±0.56 m vs. 1.57±0.77 m, respectively) [14].
In our study, male COPD patients showed higher exercise capacity at higher degrees of airflow limitation. Data from the female COPD patients cohort study indicated that despite similar predicted FEV1 values, females exhibited significantly shorter 6-MWD than males (366±89.9 vs. 384±120.5) [15]. Therefore, sex may affect exercise capacity in COPD patients showing higher exercise capacity in male despite some degree of airflow limitation.
There is a significant relationship between hemoglobin levels and exercise capacity in COPD patients. Low hemoglobin levels can contribute to reduced exercise capacity and poor 6-MWD in COPD patients. A previous study indicated that among 105 patients with COPD (FEV1=1.3±0.6), patients with anemia (12.3%) covered a significantly shorter 6-MWD (267.9±86.7) compared to patients without anemia (373.0±122.8, p=0.001) suggesting that hemoglobin levels were independently associated with decreased exercise capacity [16]. In a cohort of 683 stable COPD patients, patients with anemia experienced significantly higher levels of dyspnea, reduced exercise capacity, and shorter median survival duration compared to their nonanemic counterparts [17]. In our study, in COPD patients with mild to moderate airflow obstruction, hemoglobin levels were found to be associated with a discrepancy between airflow limitation and 6-MWD. This may reflect that maintaining hemoglobin levels may improve exercise capacity in COPD patients. Further, a previous study indicated that the intravenous administration of iron improved exercise capacity and reduced breathlessness in COPD patients [18].
In our study, an association between DLCO and differences between the degree of airflow limitation and exercise capacity were observed in COPD patients with severe to very severe airflow limitation. Several studies have reported that DLCO is a strong predictor of exercise capacity in COPD patients. In a previous study that examined 6-MWD, spirometer, lung volume, and DLCO in 130 stable COPD patients, only DLCO was observed to be substantially linked with 6-MWD in multivariate analysis [19]. In addition, a decline of 6-MWD (>30 m) was reported to be associated with reduced DLCO regardless of similar FEV1 value [20]. Similar to our findings, these studies suggest that even severe to very severe airflow obstruction COPD patients with preserved diffusion capacity may demonstrate preserved exercise capacity.
There were some limitations to our investigation. First, some COPD patients did not complete the 6-MWD test due to dyspnea or muscle fatigue in their limbs. These patients were typically older, had a lower BMI, and experienced skeletal muscle atrophy, which could be attributed to medication use or the effects of COPD itself. Additionally, there may have been variations in the timing of the 6-MWD test among the study participants. The test is usually performed as an initial assessment for COPD, to avoid the influence of medication. However, it can also be conducted while patients are taking medications, such as bronchodilators. Consequently, the exercise performance of these patients may have been compromised.
In conclusion, this study demonstrated that the discrepancy between airflow limitation and exercise capacity in COPD patients was associated with sex, smoking status, hemoglobin levels, and lung diffusion capacity. Therefore, it suggests that exercise capacity in COPD patients may be influenced by various factors beyond airflow limitation, highlighting the importance of considering these aspects when assessing and treating patients.
Notes
Authors’ Contributions
Conceptualization: Kim TH, Kim HC. Methodology: Kim HC. Formal analysis: Kim HC. Data curation: Heo IR, Kim NY, Park JH, Yoon HY, Jung JY, Ra SW, Jung KS, Yoo KH. Software: Kim HC. Validation: Kim HC. Writing - original draft preparation: Kim TH, Kim HC. Writing - review and editing: Heo IR, Kim NY, Park JH, Yoon HY, Jung JY, Ra SW, Jung KS, Yoo KH. Approval of final manuscript: all authors.
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Funding
This work was supported by a Research Program funded by the Korea National Institute of Health (Fund CODE 2016ER670100, 2016ER670101, 2016ER670102, 2018 ER67100, 2018ER67101, 2018ER67102, 2021ER120500, 2021ER120501, and 2021ER120502).