Direct Switch from Tiotropium to Indacaterol/Glycopyrronium in Chronic Obstructive Pulmonary Disease Patients in Korea
Article information
Abstract
Background
Many chronic obstructive pulmonary disease (COPD) patients receiving monotherapy continue to experience symptoms, exacerbations and poor quality of life. This study aimed to assess the efficacy and safety of direct switch from once-daily tiotropium (TIO) 18 μg to indacaterol/glycopyrronium (IND/GLY) 110/50 μg once daily in COPD patients in Korea.
Methods
This was a randomized, open-label, parallel group, 12-week trial in mild-to-moderate COPD patients who received TIO 18 μg once daily for ≥12 weeks prior to study initiation. Patients aged ≥40 years, with predicted post-bronchodilator forced expiratory volume in 1 second (FEV1) ≥50%, post-bronchodilator FEV1/forced vital capacity <0.7 and smoking history of ≥10 pack-years were included. Eligible patients were randomized in a 1:1 ratio to either IND/GLY or TIO. The primary objective was to demonstrate superiority of IND/GLY over TIO in pre-dose trough FEV1 at week 12. Secondary endpoints included transition dyspnea index (TDI) focal score, COPD assessment test (CAT) total score, and rescue medication use following the 12-week treatment, and safety assessment.
Results
Of the 442 patients screened, 379 were randomized and 347 completed the study. IND/GLY demonstrated superiority in pre-dose trough FEV1 versus TIO at week 12 (least squares mean treatment difference [Δ], 50 mL; p=0.013). Also, numerical improvements were observed with IND/GLY in the TDI focal score (Δ, 0.31), CAT total score (Δ, −0.81), and rescue medication use (Δ, −0.09 puffs/day). Both treatments were well tolerated by patients.
Conclusion
A direct switch from TIO to IND/GLY provided improvements in lung function and other patient-reported outcomes with an acceptable safety profile in patients with mild-to-moderate airflow limitation.
Introduction
The prevalence of chronic obstructive pulmonary disease (COPD) is considerably high, affecting 251 million patients globally. In 2016, more than 3 million deaths were reported due to COPD; by 2030, it is estimated to be the third leading cause of deaths worldwide1. In Republic of Korea, the prevalence of COPD among patients aged >40 years was 13.4% in 2015, and is on the rise2,3, contributing to significant economic burden4.
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2019 strategy recommends initial treatment with a single bronchodilator—short- or long-acting for all GOLD groups depending on the disease severity5. However, many COPD patients receiving monotherapy continue to experience symptoms, exacerbations and poor quality of life, and a dual bronchodilator therapy (long-acting β2-agonist/long-acting muscarinic antagonist [LABA/LAMA]) is recommended for these patients6. In Korea, 70 to 80% of COPD patients using an inhaled mono-bronchodilator still experience the same or more symptoms despite treatment compared to the time they were first diagnosed7.
As per the Korean clinical practice guideline for COPD, LABA/LAMA fixed-dose combination is recommended for patients in group Na (modified medical research council ≥2 or COPD assessment test [CAT] ≥10, forced expiratory volume in 1 second [FEV1] ≥60% predicted and 0–1 exacerbation/yr) who have severe breathlessness or show no improvement in symptoms with monotherapy or experience exacerbations, and as first-line therapy in group Da patients (FEV1 <60% predicted and ≥2 exacerbations/yr or history of acute exacerbation of COPD related hospitalization)8.
Tiotropium (TIO) 18 μg once daily is a well established LAMA in the management of COPD, and the most widely used monobronchodilator in Korea9. Indacaterol/glycopyrronium (IND/GLY) 110/50 μg once daily is a dual LABA/LAMA bronchodilator that has demonstrated greater improvements in efficacy outcomes versus TIO 18 μg once daily in COPD patients, including those of Asian ethnicity in several phase III trials10–12.
The open-label CRYSTAL study13 evaluated a direct switch from LABA or LAMA monotherapies and LABA+inhaled corticosteroid (ICS) combinations to IND/GLY 110/50 μg once daily in COPD patients with moderate airflow limitation. The SHINE study evaluated IND/GLY 110/50 μg once daily versus TIO 18 μg once daily, monocomponents and placebo in treatment-naïve patients with moderate-to-severe COPD but with a washout period between two treatments compared to placebo11. However, there are limited data available to date specifically for a direct switch from TIO 18 μg once daily to IND/GLY 110/50 μg once daily in patients with COPD14. This study aimed to assess the efficacy and safety of a direct switch from TIO 18 μg once daily to IND/GLY 110/50 μg once daily in symptomatic COPD patients with mild-to-moderate airflow limitation from Korea.
Materials and Methods
1. Study design
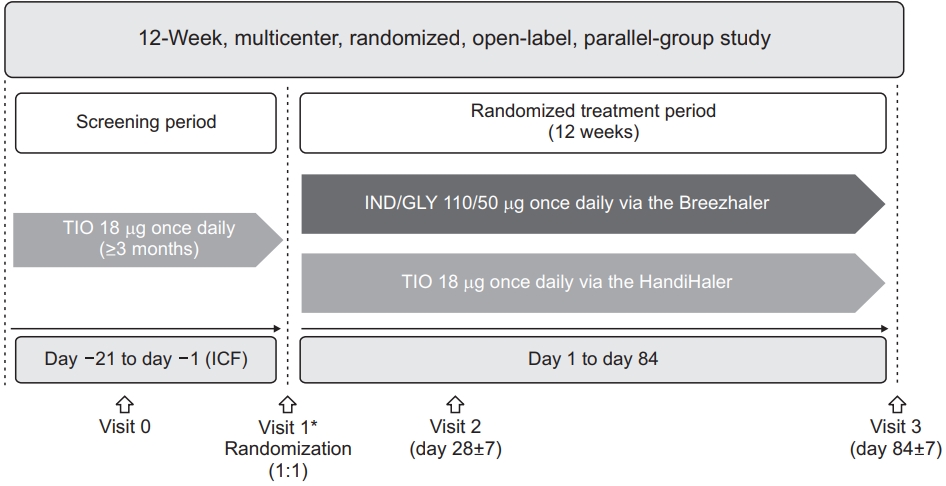
This was a randomized, multicentre, open-label, parallel group, 12-week phase IV trial in mild-to-moderate COPD patients who received TIO 18 μg once daily for at least 12 weeks prior to study initiation (ClinicalTrials.gov no. NCT02566031) (Figure 1). Eligible patients from 24 participating study centers were randomized (1:1) to receive either IND/GLY 110/50 μg once daily (delivered via the Breezhaler device, Novartis Pharma AG, Stein, Switzerland) or TIO 18 μg once daily (delivered via the HandiHaler device, Boehringer Ingelheim, Ingelheim am Rhein, Germany) during the study. Detailed study design has been published previously15. The study was approved by the institutional review boards and ethics committees (XC15 MSMV0093P) of the participating centers (Supplementary Table S1) and was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. All study participants provided written informed consent.
2. Patients
Patients aged ≥40 years with post-bronchodilator FEV1 ≥50% of predicted and post-bronchodilator FEV1/forced vital capacity <0.70 (severity of airflow limitation grade 1 or 2 as per GOLD 2015) were included. At study entry, patients were required to have a CAT score ≥10, a smoking history of ≥10 pack-years and not more than 1 COPD exacerbation in the previous 12 months prior to baseline (not leading to hospitalization). All patients included in study were on treatment with TIO 18 μg once daily in the 3 months prior to baseline. Patients with COPD exacerbation between screening and baseline visit, prior or current diagnosis of asthma or treatment with any ICS in the 3 months prior to baseline were excluded from the study. Detailed patient criteria have been published previously15.
3. Endpoints
The study was conducted to evaluate effect of IND/GLY 110/50 μg once daily versus TIO 18 μg once daily in terms of lung function, symptom burden, breathlessness, rescue medication use and safety in patients with mild-to-moderate COPD. The primary endpoint of the study was to demonstrate superiority of IND/GLY 110/50 μg once daily versus TIO 18 μg once daily in pre-dose trough FEV1 following 12 weeks of treatment. Secondary endpoints included improvement in pre-dose trough FEV1 following 4 weeks of treatment, transition dyspnea index (TDI) focal score at week 12, CAT total score at week 12, rescue medication use with IND/GLY 110/50 μg once daily versus TIO 18 μg over 12 weeks. Safety and tolerability were evaluated over the study period in terms of adverse events (AEs) and serious adverse events (SAEs) with IND/GLY 110/50 μg once daily and TIO 18 μg once daily.
4. Statistical analysis
Analysis of primary endpoint and all efficacy endpoints were performed on the full analysis set (FAS). The FAS included all randomized patients who received at least one dose of study treatment and had at least one evaluable post-baseline assessment. An analysis of covariance (ANCOVA) was used for the primary endpoint with treatment, smoking status as fixed effects, baseline trough FEV1 as a covariate and center as random effect. To demonstrate superiority, 95% confidence interval (CI) associated with least squares mean treatment difference in trough FEV1 with IND/GLY versus TIO should fall entirely to the right of (greater than) 0 mL, and p-value should be <0.05. A sample size of 173 patients in each treatment arm were required to achieve 80% power on a 2-sided test with 5% level of significance; assuming a dropout rate of 11%, approximately 389 patients were planned to be enrolled in the study; however, 379 patients were randomized (IND/GLY, 189; TIO, 190). Based on the results from the SHINE study10, it was assumed that the estimated treatment difference between IND/GLY and TIO is 80 mL and corresponding standard deviation is 271 mL.
Mean daily number of puffs of rescue medication use was analyzed using a negative binomial regression model. Percentage of days with no rescue medication use, TDI focal score and CAT score were analyzed using the same ANCOVA model as for the primary analysis. Adjusted least square means, point estimate of difference between treatment means, 95% CI and p-values were presented for efficacy variables. Proportion of patients with a clinically important improvement of at least 100 mL in trough FEV1, 1-unit increase in the TDI focal score and 2 points decrease were analyzed using logistic regression, and presented with odds ratio and associated 95% CI and p-values. All safety endpoints were summarised by treatment for all patients in the safety set, which included all patients who received at least one dose of the study treatment. Additional details on statistical analysis are included in Supplementary Material S1.
Results
1. Patients
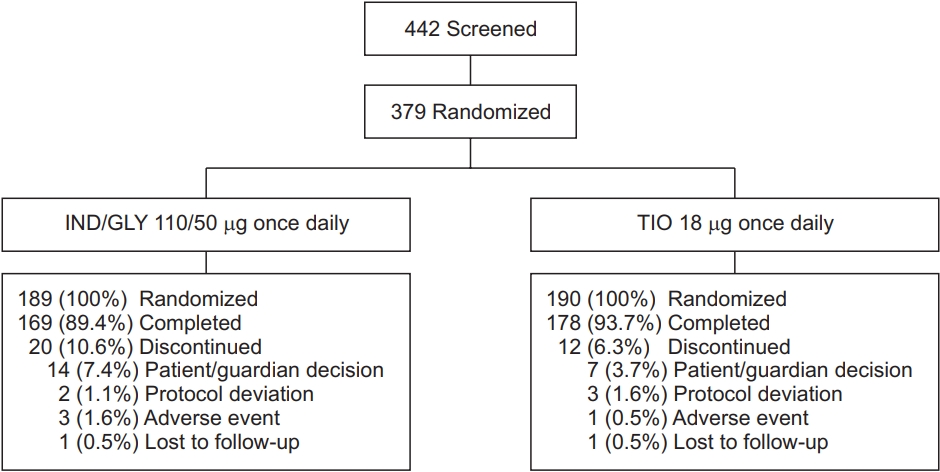
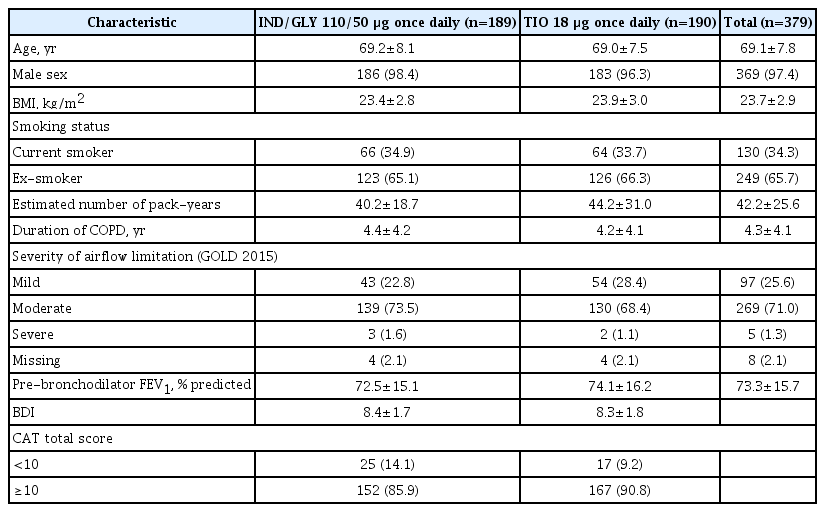
Of the 442 patients screened, 379 completed the screening and were randomized to receive either IND/GLY 110/50 μg once daily (n=189) or TIO 18 μg once daily (n=190); both treatments were administered open-labelled. In total, 347 patients completed the study phase (Figure 2). The rate of discontinuation was numerically higher in IND/GLY group (10.6%) versus TIO group (6.3%). Overall, 361 patients were included in FAS (IND/GLY, 177; TIO, 184), 377 in safety set (IND/GLY, 189; TIO, 188) and 287 in per protocol set (PPS) (IND/GLY, 138; TIO, 149). Baseline demographic and clinical characteristics of the patients were broadly similar between IND/GLY and TIO groups (Table 1). Majority of patients in both the groups were men, had mean age of ~69 years and were ex-smokers. The study population was representative of intended target population with 71% patients experiencing moderate airflow limitation and 25.6% patients with mild airflow limitation.
2. Lung function
The mean (±standard deviation) change from baseline in pre-dose trough FEV1 was greater for IND/GLY 110/50 μg once daily versus TIO 18 μg once daily at week 4 (0.08±0.201 L vs. 0.02±0.152 L) and week 12 (0.07±0.181 L vs. 0.01±0.185 L). IND/GLY 110/50 μg once daily demonstrated superiority versus TIO 18 μg once daily with a significant improvement in trough FEV1 at week 12; the 95% CI values associated with treatment difference were greater than 0 mL with p<0.05 (Figure 3).
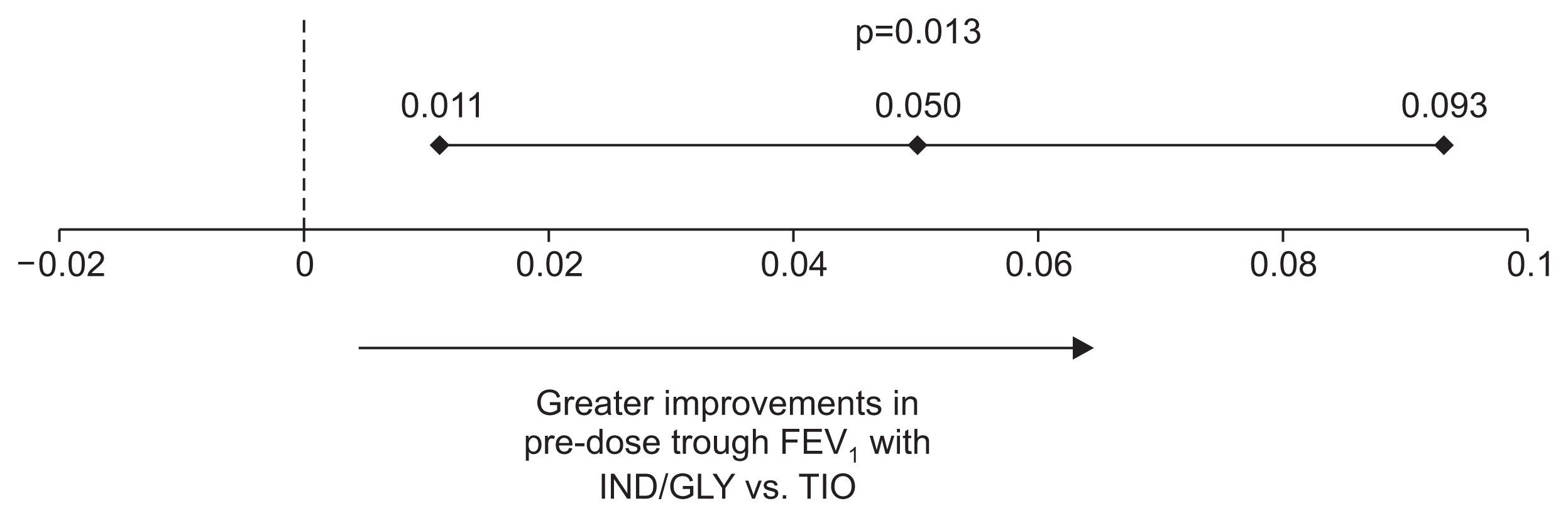
Treatment difference between IND/GLY and TIO in pre-dose trough FEV1 (L) at week 12 (FAS). Data are presented as least squares mean treatment difference and associated 95% CI values. FEV1: forced expiratory volume in 1 second; IND/GLY: indacaterol/glycopyrronium 110/50 μg once daily; TIO: tiotropium 18 μg once daily; FAS: full analysis set.
The proportion of patients achieving minimal clinically important difference (MCID) of ≥100 mL increase in pre-dose trough FEV1 was significantly greater with IND/GLY 110/50 μg once daily (42.2%), compared with TIO 18 μg once daily (26.4%) after 12 weeks of treatment (Figure 4).
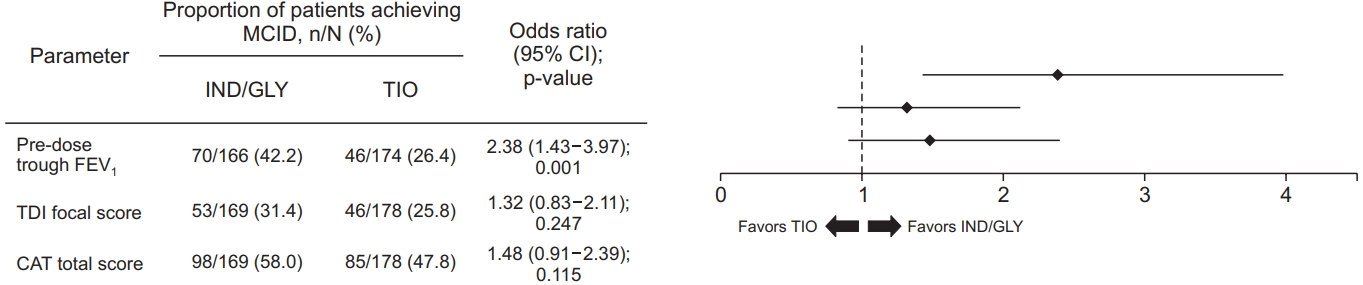
Patients achieving MCID in pre-dose trough FEV1, TDI focal score and CAT total score with IND/GLY and TIO at week 12. Data are presented as odds ratio and associated 95% CI values. MCID: minimal clinically important difference; n: number of patients achieving MCID; N: total number of patients analyzed; IND/GLY: indacaterol/glycopyrronium 110/50 µg once daily; TIO: tiotropium 18 µg once daily; CI: confidence interval; FEV1: forced expiratory volume in 1 second; TDI: transition dyspnea index; CAT: chronic obstructive pulmonary disease assessment test.
Improvement in pre-dose trough FEV1 were also significant with IND/GLY 110/50 μg once daily versus TIO 18 μg once daily at week 4 (p=0.003) (Supplementary Figure S1). In the PPS population also, IND/GLY 110/50 μg once daily demonstrated improvement in pre-dose trough FEV1 versus TIO 18 μg once daily at week 12 (p=0.088) (Supplementary Figure S2).
3. Dyspnea
At week 12, IND/GLY 110/50 μg once daily demonstrated greater improvement in TDI focal score, compared with TIO 18 μg once daily; however, the difference was not significant (Supplementary Figure S3). The proportion of patients achieving MCID of ≥1-unit improvement in TDI focal score was greater with IND/GLY 110/50 μg once daily (31.4%) than TIO 18 μg once daily (25.8%) after 12 weeks of treatment (Figure 4).
4. Symptoms
At week 12, patients treated with IND/GLY 110/50 μg once daily had lower CAT total score versus TIO 18 μg once daily although it was not statistically significant (Supplementary Figure S4). The proportion of patients achieving MCID of ≥2-point decrease in CAT total score was numerically greater with IND/GLY 110/50 μg once daily (58.0%), compared with TIO 18 μg once daily (47.8%) after 12 weeks of treatment (Figure 4).
5. Rescue medication use
There was no statistical difference between IND/GLY 110/50 μg once daily and TIO 18 μg once daily in the number of puffs of rescue medication use during the study period (Supplementary Figure S5). The mean percentage of days with no rescue medication use was comparable between IND/GLY 110/50 μg once daily (29.0%) and TIO 18 μg once daily (28.6%) over 12 weeks of treatment.
6. Safety
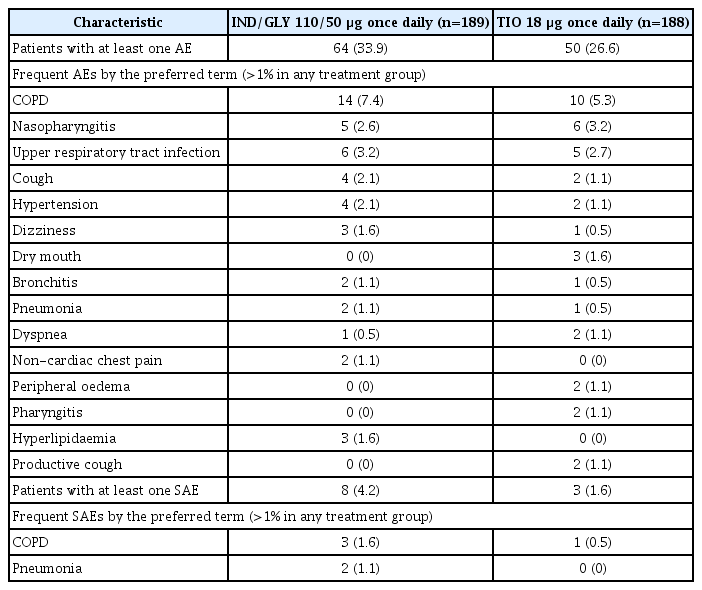
The overall incidence of AEs (including COPD exacerbations) was numerically higher in the IND/GLY 110/50 μg once daily group (33.9%), compared with the TIO 18 μg once daily group (26.6%) at week 12; however, the difference was not statistically significant (p=0.125). COPD worsening was the most frequent AE reported in 7.4% and 5.3% patients in IND/GLY 110/50 μg once daily and TIO 18 μg once daily, respectively. AEs that were suspected to be related to the study drug, based on investigator assessment, were reported more in the IND/GLY arm (6.3%) versus the TIO arm (1.1%). Incidence of SAEs with IND/GLY 110/50 μg once daily was comparable with TIO 18 μg once daily at week 12 (Table 2). A total of three patients in the IND/GLY group and one patient in the TIO group discontinued study treatment due to AEs. No deaths were reported during the study or the follow-up period.
Discussion
In this 12-week, open-label, randomized trial, a switch from TIO 18 μg once daily to IND/GLY 110/50 μg once daily led to significant improvement in pre-dose trough FEV1 in patients with mild-to-moderate COPD, compared with continued treatment with TIO 18 μg once daily The study met its primary endpoint of superiority in improvement in pre-dose trough FEV1 with IND/GLY 110/50 μg once daily versus TIO 18 μg once daily A trend of improvement was also observed in dyspnea, symptoms and rescue medication use with IND/GLY versus TIO; however, the differences were not significant. Safety profile was also comparable between the two treatment arms.
Although the efficacy and safety of IND/GLY has been evaluated versus TIO in randomized clinical trials, evidence is limited for comparison of these treatments which can mimic real-life clinical practice. This study adds to the real-world evidence generated through POWER14, CRYSTAL13, and FLASH16 studies, which showed greater improvements with IND/GLY on direct switch from other COPD treatments (LABA or LAMA monotherapies, TIO, salmeterol/fluticasone and LABA+ICS). In the present study, improvement in pre-dose trough FEV1 observed with IND/GLY versus TIO at week 12 (Δ, 50 mL; p=0.013) was comparatively less than that observed in the SHINE study (Δ, 80 mL; p<0.001) at week 2611. Notwithstanding the magnitude of difference, our study still showed superiority of IND/GLY. It should be noted that patients enrolled in present study were directly switched from TIO to IND/GLY (or continued treatment TIO) without any washout period; unlike the SHINE study. So carry-over effect of previous treatment should be considered while interpreting the results.
Another aspect that needs to be considered is the older age and low body mass index (BMI) of patients from the present study (mean age, 69.1 years; BMI, 23.7 kg/m2) compared with the SHINE study (mean age, 63.9 years; BMI, 25.9 kg/m2), which could have contributed to this difference. It is well known that low BMI and older age are associated with poor lung function, health status and dyspnea symptoms17. In the pooled analysis of SHINE and ARISE study in Japanese patients5 with comparable age and BMI to present study, improvement in trough FEV1 with IND/GLY was 70 mL (p=0.001) at week 12, which was in line with the result from our study. In the CRYSTAL study, IND/GLY provided greater improvements in trough FEV1 at week 12 versus LABA or LAMA monotherapy (Δ, 101 mL; p<0.0001); the magnitude of which was greater, compared with LAMA (TIO) in present study. Similar differences were also observed for other evaluated endpoints. In the POWER study, a direct switch from TIO to IND/GLY provided significant improvements in lung function and patient-reported outcomes in patients with moderate-to-severe COPD. IND/GLY demonstrated an improvement of 172 mL in trough FEV1 from baseline to week 16 in COPD patients who switched from TIO14. In the present study, change from baseline in trough FEV1 was 70 mL at week 12 with IND/GLY, which was lower than that observed in the POWER study. Difference in study design, ethnic difference in patient population should be considered while comparing results from these studies. IND/GLY and TIO demonstrated an acceptable safety profile, which was comparable to that observed in randomized clinical trials9,18.
Results from this study support treatment recommendations from GOLD 2019 and Korean guidelines to switch patients uncontrolled on LAMA monotherapy to LABA/LAMA combination. The current study has certain strengths and limitations. The most important strength is that by way of a direct switch we compared the efficacy of dual bronchodilation with IND/GLY 110/50 μg once daily versus TIO 18 μg once daily in a clinical practice setting, to answer a clinically relevant question. The study also included patients with mild airflow limitation who are generally not included in COPD clinical trials, and there is a lack of evidence of therapeutic efficacy in these patients. The study generated robust evidence in Korean COPD patients by virtue of its sample size. A limitation of this study was that due to open-label nature of such trials, there may be potential bias in reporting of patient-reported outcomes. Second, the study duration was 12 weeks and may not be sufficient to be fully reflective of the long-term effectiveness of treatments.
A direct switch from TIO 18 μg once daily to IND/GLY 110/50 μg once daily provided significant improvement in pre-dose trough FEV1 in patients with mild-to-moderate airflow limitation from Republic of Korea. Numerical improvements were also observed in TDI focal score, CAT score, and rescue medication use with IND/GLY 110/50 μg once daily versus TIO 18 μg once daily. Safety profile of the two treatment groups was comparable. Data from this study add to the existing evidence supporting greater efficacy of IND/GLY over TIO in patients with COPD.
Supplementary Material
Supplementary material can be found in the journal homepage (http://www.e-trd.org).
Supplementary Table S1. List of participating study centers in Korea.
trd-2020-0109-suppl1.pdfSupplementary Figure S1. Treatment difference between IND/GLY and TIO in pre-dose trough FEV1 (L) at at week 4 (FAS).
trd-2020-0109-suppl2.pdfSupplementary Figure S2. Treatment difference between IND/GLY and TIO in pre-dose trough FEV1 (L) at at week 12 (PPS).
trd-2020-0109-suppl3.pdfSupplementary Figure S3. Treatment difference between IND/GLY and TIO in TDI focal score at week 12 (FAS).
trd-2020-0109-suppl4.pdfSupplementary Figure S4. Treatment difference between IND/GLY and TIO in CAT total score at week 12 (FAS).
trd-2020-0109-suppl5.pdfSupplementary Figure S5. Rescue medication use with IND/GLY and TIO over 12 weeks (FAS).
trd-2020-0109-suppl6.pdfSupplementary Material S1. Statistical analysis.
trd-2020-0109-suppl7.pdfAcknowledgements
The authors thank investigators from all participating study centers for their contribution to conduct of study. The authors also acknowledge Rajendra Prasad for expert review of this draft. Medical writing support was provided by Vatsal Vithlani and Rahul Lad (professional medical writers from Novartis) under author’s guidance. The article was prepared in accordance with the third edition of Good Publication Practice guidelines.
Notes
Initial data were presented at the 23rd Congress of Asian Pacific Society of Respirology, November 29–December 2 2018, Taipei, Taiwan and 126th Fall Congress of Korean Academy of Tuberculosis and Respiratory Disease (KATRD), November 8–9, 2018, Seoul, Korea.
Authors’ Contributions
Conceptualization: Lee SH, Rhee CK, Yoo K, Park JW, Yong SJ, Kim J, Lee T, Lim SY, Lee JH, Park HY, Moon M, Jung KS. Methodology: Lee SH, Rhee CK, Yoo K, Park JW, Yong SJ, Kim J, Lee T, Lim SY, Lee JH, Park HY, Moon M, Jung KS. Formal analysis: Moon M. Investigation: Lee SH, Rhee CK, Yoo K, Park JW, Yong SJ, Kim J, Lee T, Lim SY, Lee JH, Park HY, Jung KS. Writing - review and editing: Lee SH, Rhee CK, Yoo K, Park JW, Yong SJ, Kim J, Lee T, Lim SY, Lee JH, Park HY, Moon M, Jung KS. Approval of final manuscript: all authors.
Conflicts of Interest
Rhee CK received consulting/lecture fees from MSD, Astra-Zeneca, GSK, Novartis, Takeda, Mundipharma, Boehringer-Ingelheim, Teva, and Bayer. Lee SH, Yoo K, Park JW, Yong SJ, Kim J, Lee T, Lim SY, Lee JH, Park HY and Jung KS report no conflict for interest in relation to this work. Moon M is an employee of Novartis. Jung KS serves as editor-in-chief of the Tuberculosis of Respiratory Diseases, but has no role in the decision to publish this article.
Funding
The study was funded by Novartis Korea Ltd., Seoul, South Korea.