 |
 |
| Tuberc Respir Dis > Volume 74(3); 2013 > Article |
|
Abstract
The presence of epidermal growth factor receptor (EGFR) mutation is a prognostic and predictive marker for EGFR-tyrosine kinase inhibitor (TKI) therapy. However, inevitably, relapse occurs due to the development of acquired resistance, such as T790M mutation. We report a case of repeated responses to EGFR-TKIs in a never-smoked woman with adenocarcinoma. After six cycles of gemcitabine and cisplatin, the patient was treated by gefitinib for 4 months until progression. Following the six cycles of third-line pemetrexed, gefitinib retreatment was initiated and continued with a partial response for 6 months. After progression, she was recruited for an irreversible EGFR inhibitor trial, and the time to progression was 11 months. Although EGFR direct sequencing on the initial diagnostic specimen revealed a wild-type, we performed a rebiopsy from the progressed subcarinal node at the end of the trial. The result of peptide nucleic acid clamping showed L858R/L861Q.
Epidermal growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI) has improved the outcome of advanced non-small cell lung cancer (NSCLC). EGFR-TKIs have higher response rates in Asian patients, females, non-smokers, and those with adenocarcinoma histology1-3. The efficacy of EGFR-TKIs is associated with the activation of EGFR mutations2,3. However, most patients who respond to EGFR-TKIs eventually experience progression after approximately 10-14 months4.
Here, we report a case of three repeated favorable responses to EGFR-TKIs including gefitinib retreatment and irreversible EGFR inhibitor in a patient with stage IV adenocarcinoma.
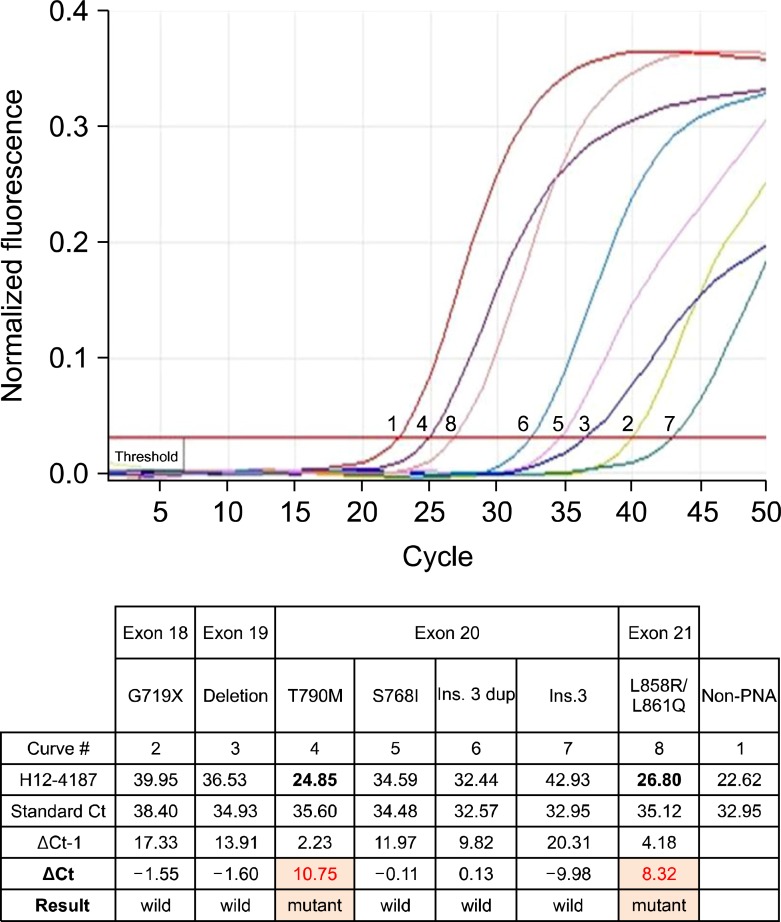
In December 2008, a 43-year-old Korean woman was admitted to our hospital with a 3-month history of dry cough and dyspnea. The patient had never smoked and had no recent history of travel or use of medications. Physical examination revealed some crackles on the left upper lung. Laboratory findings were within normal range, except for elevations of white blood cell count (26,600/mm3) and C-reactive protein (7.6 mg/dL). Chest radiograph (Figure 1) and computed tomography (CT) scans showed diffuse consolidations and ground glass opacities mixed with multiple hematogenous metastases in the left upper lobe (LUL) and left lower lobe. Pathologic results of transbronchial lung biopsy from the LUL showed a well-differentiated adenocarcinoma. We performed direct DNA sequencing5 for EGFR mutations in exon 18, 19, 20, and 21 with this specimen. The result indicated wild-type EGFR. First-line chemotherapy with gemcitabine and cisplatin was started on December 2008. After 6 cycles, second-line treatment with gefitinib 250 mg/day was initiated in May 2009 because of progressive disease. A follow-up CT showed partial response (Figure 2A, B). But, the time-to-progression of gefitinib was only 4 months (Figure 2C), so she received third-line pemetrexed for 6 cycles. After that the CT scan in July 2010 revealed aggravated pulmonary metastatic nodules, and we enrolled the patient in an ongoing clinical trial6 for evaluation of gefitinib reatreatment in advanced NSCLC patients who were controlled previously with gefitinib. The patient displayed marked improvement in symptoms and radiologic findings (Figure 2D, E). We attempted to obtain a gefitinib-resistant tumor biopsy sample at the end of this trial, but could not perform EGFR mutation analysis because of insufficient specimen. After 6 months, there were more progressed LUL nodule and hematogenous metastases (Figure 2F). On the suspicion of clinically defined acquired resistance to EGFR-TKI, we recruited the patient to a phase II trial with PF-00299804 (PF-00299804 in treating patients with stage IIIB or stage IV NSCLC that has not responded to standard therapy for advanced or metastatic cancer, ClinicalTrials.gov number, NCT0100-0025). In April 2011, the patient was started on PF-00299804 as fifth-line therapy. A radiologically evident partial response occurred once more (Figure 2G, H). The patient continued the experimental drug for 11 months before newly developed subcarinal lymphadenopathy was detected by CT imaging in March 2012 (Figure 2I). Endobronchial ultrasonography-guided transbronchial needle aspiration biopsy confirmed metastaic adenocarinoma. An EGFR mutation test with this specimen using the peptide nucleic acid (PNA) clamping method5,6 revealed two mutations in exon 21 (L858R/L861Q) and exon 20 (T790M) (Figure 3). With the progressive disease, sixth-line docetaxel chemotherapy was started beginning in April 2012.
To the best of our knowledge, this is the first case report of repeated partial responses to gefitinib retreatment and subsequent irreversible EGFR inhibitor in a patient with advanced adenocarcinoma in Korea. Recent retrospective and prospective reports have suggested the possibility that retreatment of EGFR-TKI might be useful for initial responders following a drug holiday6-8. Oh et al.6 reported a disease control rate of 65.2% in advanced NSCLC patients with retreatment of gefitinib after prior gefitinib treatment evidenced partial response or stable disease. However, as in the present case, despite repetitive responses to EGFR-TKIs, the development of secondary resistance inevitably leads to treatment failure. Recently, several second-generation irreversible EGFR inhibitors have been developed with a specific focus on T790M to overcome acquired resistance of EGFR-TKI. Clinical studies have addressed the benefits of irreversible EGFR inhibitors such as afatinib or PF-002998049-11. PF-00299804 is an oral, irreversible small-molecule inhibitor of HER1, HER2, and HER4. Our case also showed clinical favorable response to PF-00299804 with duration of 11 months.
However, as in our patient, other mechanisms can lead to eventually relapse under irreversible EGFR inhibitor therapy. Ercan et al.12 suggested that the amplification of T790M causes resistance to an irreversible EGFR inhibitor both in vitro and using a xenograft model in vivo. They demonstrated that although irreversible EGFR inhibitors may be transiently effective against cancers harboring EGFR T790M, clones harboring amplified T790M will rapidly emerge in vitro and in vivo through selection of pre-existing T790M amplified or high-expressing clones leading to clinical drug resistance. This findings show that EGFR T790M is a common resistance mechanism to both reversible, and when amplified, the irreversible EGFR inhibitors. In our case, the response duration of irreversible EGFR inhibitor was 11 months which was unusually longer than those of initial gefitinib (for 4 months) and second round gefitinib (for 6 months) treatments. Recently an alternative approach is to develop novel strategies, instead of irreversible EGFR inhibitors, to inhibit EGFR and/or EGFR signaling, including the use of inhibitors of HSP90 and/or EGFR downstream signaling (such as phosphatidylinositol 3-kinase and MEK inhibitors) as these resistant cancers still remain dependent on EGFR signaling for their growth12. And multiple target therapy, such as multiple kinase inhibition (e.g., EGFR and c-met) or a combination of EGFR-TKI with chemotherapy or vertical inhibition by small molecules and antibodies will be needed to overcome secondary EGFR-TKI resistance for the near future.
Another interesting point of the present case is the suggestion that PNA clamping is a more sensitive method for the detection of EGFR mutations, especially with a specimen containing a low proportion of tumor cells. Some clinical data have suggested that PNA clamping is a simple procedure that is more sensitive to direct sequencing for the detection of EGFR mutations5,13. Although EGFR gene mutation analysis by direct sequencing on initial bronchoscopic lung biopsy specimen revealed wild-type EGFR, rebiopsy specimen by the PNA clamping method after failure of irreversible EGFR inhibitor therapy revealed L858R/L861Q and T790M mutations. Because of repeated favorable responses to initial gefitinib and second round gefitinib, we suggest that the result of the initial EGFR mutation test was a false negative due to insufficient specimen. Although we could not perform an EGFR mutation test at the time of progression after gefitinib retreatment, the patient had another partial response with irreversible EGFR inhibitor, which is believed to overcome secondary resistance to reversible EGFR-TKI related with T790M mutation. So, we suggest that the patient had acquired T790M mutation in addition to the original L858R/L861Q after gefitinib retreatment.
We report an adenocarcinoma case responded three times to reversible and irreversible EGFR-TKIs. This case show that scheduled 'drug holidays' or intermittent therapy may benefit for prolonged lung cancer control.
References
1. Kim KS, Jeong JY, Kim YC, Na KJ, Kim YH, Ahn SJ, et al. Predictors of the response to gefitinib in refractory non-small cell lung cancer. Clin Cancer Res 2005;11:2244-2251. PMID: 15788673.


2. Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129-2139. PMID: 15118073.


3. Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-957. PMID: 19692680.


4. Nguyen KS, Kobayashi S, Costa DB. Acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancers dependent on the epidermal growth factor receptor pathway. Clin Lung Cancer 2009;10:281-289. PMID: 19632948.



5. Kim HJ, Lee KY, Kim YC, Kim KS, Lee SY, Jang TW, et al. Detection and comparison of peptide nucleic acid-mediated real-time polymerase chain reaction clamping and direct gene sequencing for epidermal growth factor receptor mutations in patients with non-small cell lung cancer. Lung Cancer 2012;75:321-325. PMID: 21930325.


6. Oh IJ, Ban HJ, Kim KS, Kim YC. Retreatment of gefitinib in patients with non-small-cell lung cancer who previously controlled to gefitinib: a single-arm, open-label, phase II study. Lung Cancer 2012;77:121-127. PMID: 22333554.


7. Watanabe S, Tanaka J, Ota T, Kondo R, Tanaka H, Kagamu H, et al. Clinical responses to EGFR-tyrosine kinase inhibitor retreatment in non-small cell lung cancer patients who benefited from prior effective gefitinib therapy: a retrospective analysis. BMC Cancer 2011;11:1PMID: 21194487.




8. Yokouchi H, Yamazaki K, Kinoshita I, Konishi J, Asahina H, Sukoh N, et al. Clinical benefit of readministration of gefitinib for initial gefitinib-responders with non-small cell lung cancer. BMC Cancer 2007;7:51PMID: 17374153.




9. Engelman JA, Zejnullahu K, Gale CM, Lifshits E, Gonzales AJ, Shimamura T, et al. PF00299804, an irreversible pan-ERBB inhibitor, is effective in lung cancer models with EGFR and ERBB2 mutations that are resistant to gefitinib. Cancer Res 2007;67:11924-11932. PMID: 18089823.


10. Miller VA, Hirsh V, Cadranel J, Chen YM, Park K, Kim SW, et al. Afatinib versus placebo for patients with advanced, metastatic non-small-cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (LUX-Lung 1): a phase 2b/3 randomised trial. Lancet Oncol 2012;13:528-538. PMID: 22452896.


11. Doebele RC, Oton AB, Peled N, Camidge DR, Bunn PA Jr. New strategies to overcome limitations of reversible EGFR tyrosine kinase inhibitor therapy in non-small cell lung cancer. Lung Cancer 2010;69:1-12. PMID: 20092908.


12. Ercan D, Zejnullahu K, Yonesaka K, Xiao Y, Capelletti M, Rogers A, et al. Amplification of EGFR T790M causes resistance to an irreversible EGFR inhibitor. Oncogene 2010;29:2346-2356. PMID: 20118985.




13. Han HS, Lim SN, An JY, Lee KM, Choe KH, Lee KH, et al. Detection of EGFR mutation status in lung adenocarcinoma specimens with different proportions of tumor cells using two methods of differential sensitivity. J Thorac Oncol 2012;7:355-364. PMID: 22157369.


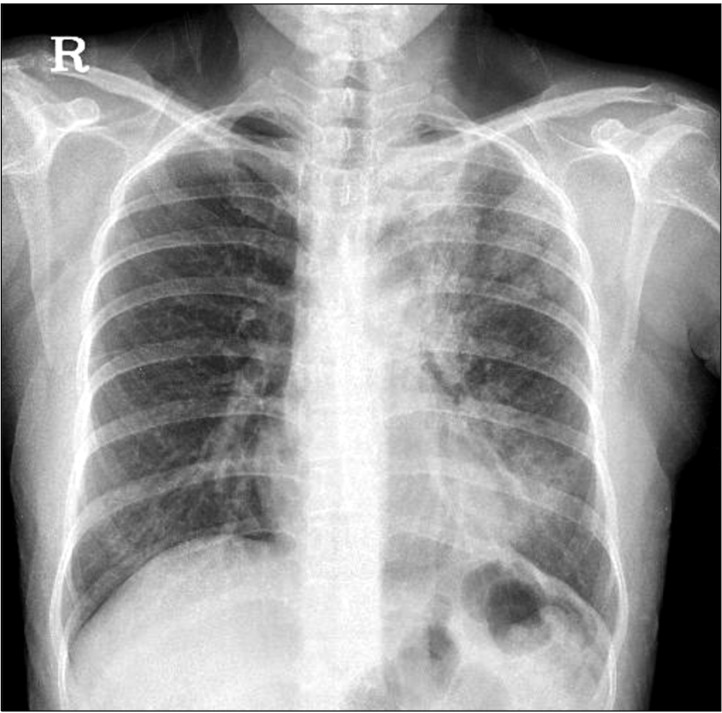
Figure┬Ā1
Initial chest radiography shows diffuse patchy consolidations with geographic ground glass opacities in the left upper and lower lung fields.
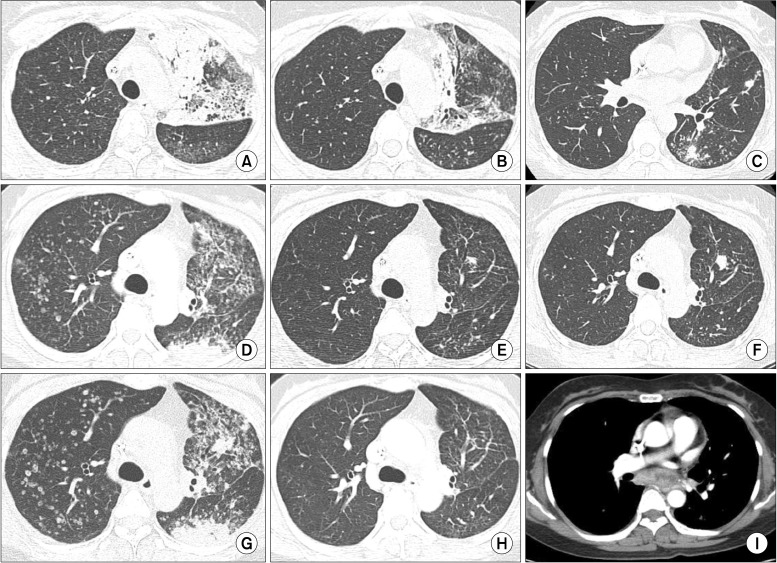
Figure┬Ā2
Computed tomography scans are displayed as the timings of (A, D, G) baseline before treatments, (B, E, H) 1-month follow-up after treatments, and (C, F, I) end of treatments. After sixth cycle of gemcitabine and cisplatin, (A) multiple consolidations were developed in left upper lobe and (B) were improved by initial gefitinib treatment. (C) Newly appeared metastatic nodules were shown in left lower lobe after 4 months. Followed by sixth cycle of pemetrexed, (D) multiple metastatic nodules were prominent in both lungs. (E) These lesions were improved by gefitinib retreatment, but (F) residual left upper lobar nodule and tiny metastases were progressed 6 months later. (G) Both lung nodules were markedly aggravated 1 month after gefitinib retreatment. (H) Dramatic response was shown after PF-00299804 trial, but metastatic subcarinal lymphadenopathy was noted in 11-month follow-up scan.


 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Print
Print Download Citation
Download Citation




