 |
 |
| Tuberc Respir Dis > Volume 85(2); 2022 > Article |
|
Abstract
Background
Methods
Results
Conclusion
Notes
Authors’ Contributions
Conceptualization: Chung MP, Jegal Y, Park JS, Jeong SH, Song JW, Kim SY. Data collection: Jegal Y, Park JS, Kim SY, Yoo H, Jeong SH, Song JW, Lee JH, Lee HL, Choi SM, Kim YW, Kim YH, Choi HS, Lee J, Uh ST, Kim TH, Kim SH, Lee WY, Kim YH, Lee HK, Lee EJ, Heo EY, Yang SH, Kang HK, Chung MP. Formal analysis: Jegal Y, Park JS, Yoo H, Choi SM, Choi HS, Lee J, Lee EJ, Kang HK. Supervision: Chung MP, Kim YW, Uh ST. Writing - original draft preparation: Jegal Y, Park JS. Writing - review and editing: Chung MP, Kim YH, Lee HL. Approval of final manuscript: all authors.
Acknowledgments
Fig. 1.
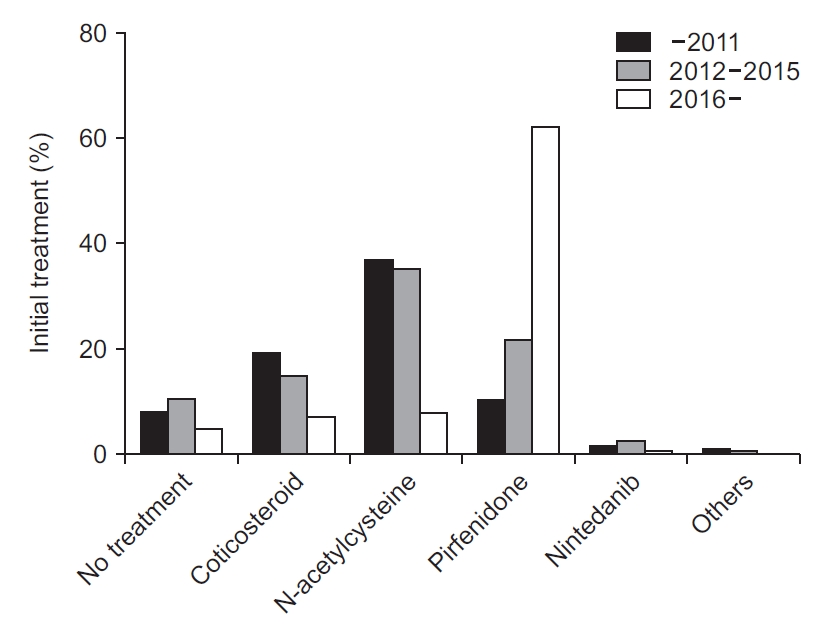
Fig. 2.

Fig. 3.

Table 1.
Table 2.
Table 3.
Values are presented as number (%) or mean±SD.
mMRC: modified Medical Research Council dyspnea scale; PaO2: arterial partial pressure of oxygen; PaCO2: arterial partial pressure of carbon dioxide; FVC: forced vital capacity; FEV1: forced expiratory volume in 1 second; DLco: diffusing capacity for carbon monoxide; CT: computed tomography; UIP: usual interstitial pneumonia; GGO: ground glass opacity; GAP: gender-age-physiology.
Table 4.
| Treatment | No. (%) |
|---|---|
| No treatment | 187 (8.7) |
| Corticosteroid | 292 (13.7) |
| N-acetylcysteine | 594 (27.8) |
| Pirfenidone | 683 (31.9) |
| Nintedanib | 46 (2.2) |
| Others | 17 (0.8) |
| Total | 1,819 |
Table 5.
| No treatment (n=33) | Antifibrotics (n=420) | Non-antifibrotics (n=77) | p-value | |
|---|---|---|---|---|
| Age | 73.4±6.6 | 70.8±8.0 | 69.6±9.1 | 0.073 |
| Sex (male:female) | 28:5 | 350:70 | 62:15 | 0.799 |
| FVC (L) | 3.19±0.82 | 2.83±0.74* | 2.78±0.91* | 0.028 |
| FVC % predicted | 83.9±17.4 | 73.0±13.7* | 73.1±20.8* | <0.001 |
| FEV1 (L) | 2.37±0.58 | 2.23±0.52 | 2.16±0.65 | 0.199 |
| FEV1 %predicted | 91.9±81.0 | 83.8±14.8* | 81.0±22.0* | 0.006 |
| DLco (mL/mm Hg/min) | 12.50±5.18 | 11.21±4.97 | 11.54±4.47 | 0.341 |
| DLco % predicted | 70.6±24.6 | 59.3±18.1* | 60.5±21.8* | 0.006 |
Table 6.
References
- TOOLS
-
METRICS

- ORCID iDs
-
Yangjin Jegal

https://orcid.org/0000-0002-1935-7240Jong Sun Park

https://orcid.org/0000-0003-3707-3636Man Pyo Chung

https://orcid.org/0000-0002-5548-0764 - Funding Information
-
Korean Academy of Tuberculosis and Respiratory Diseases
KATRD-S-2013-1 - Related articles


 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Data Sharing Statement
Data Sharing Statement Full text via DOI
Full text via DOI Print
Print Download Citation
Download Citation



