 |
 |
| Tuberc Respir Dis > Volume 87(2); 2024 > Article |
|
Abstract
Background
Methods
Results
Notes
Authors’ Contributions
Conceptualization: Rawat J, Jangpangi DS. Methodology: Kumar A, Mrigpuri P. Formal analysis: Singh AP, Bhatt R. Data curation: Kumar A, Mrigpuri P. Software: Kumar A, Mrigpuri P, Bhatt R. Validation: Rawat J, Jangpangi DS, Mrigpuri P. Investigation: Kumar A, Mrigpuri P, Singh AP. Writing - original draft preparation: Rawat J, Kumar A, Mrigpuri P. Writing - review and editing: Kumar A, Mrigpuri P, Bhatt R. Approval of final manuscript: all authors.
Acknowledgments
Fig. 1.
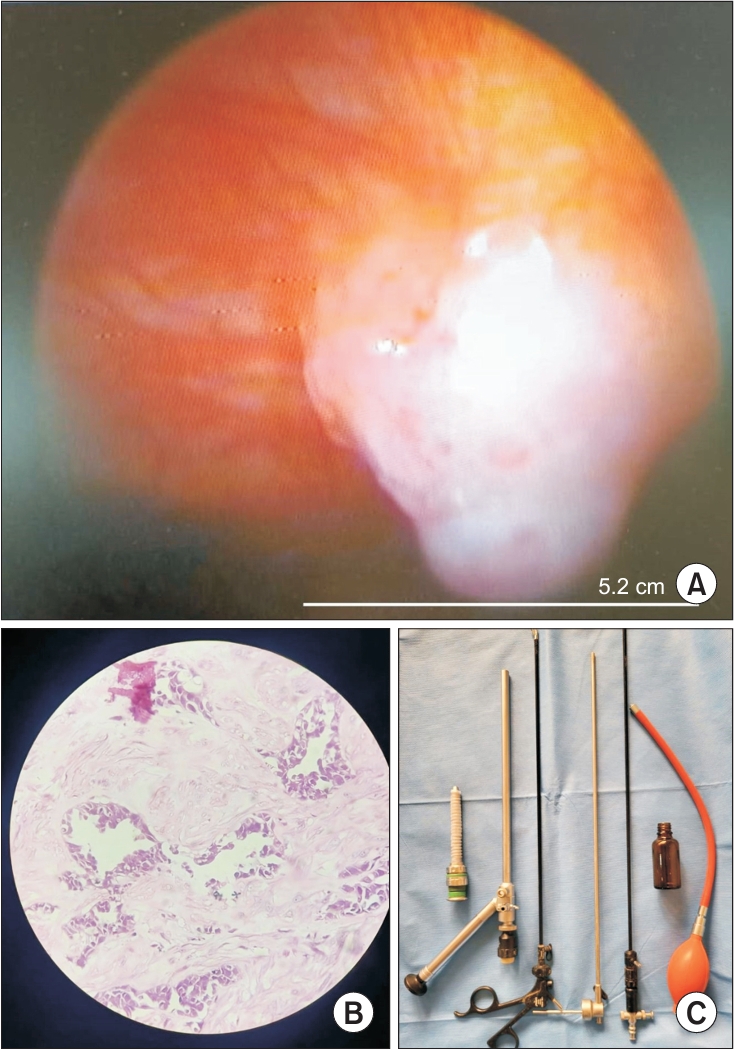
Table 1.
Table 2.
Table 3.
References
- TOOLS
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 678 View
- 56 Download
- ORCID iDs
-
Jagdish Rawat

https://orcid.org/0009-0004-9769-6514Anil Kumar

https://orcid.org/0000-0002-6461-9115 - Related articles
-
LDH Isoenzyme Pattern in Malignant Pleural Effusion1990 March;37(1)
The Role of Bronchoscopy in Determining the Etiology of Pleural Effusion.1998 April;45(2)


 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Data Sharing Statement
Data Sharing Statement Full text via DOI
Full text via DOI Print
Print Download Citation
Download Citation



