 |
 |
| Tuberc Respir Dis > Volume 87(1); 2024 > Article |
|
Abstract
Background
Methods
Results
Notes
AuthorsŌĆÖ Contributions
Conceptualization: Nyanti LE, Huan NC. Methodology: all authors. Formal analysis: Nyanti LE, Abd Rahim MA. Data curation: Nyanti LE, Abd Rahim MA. Writing - original draft preparation: Nyanti LE, Huan NC. Writing - review and editing: all authors. Approval of final manuscript: all authors.
Acknowledgments
Supplementary Material
Supplementary┬ĀTable┬ĀS1.
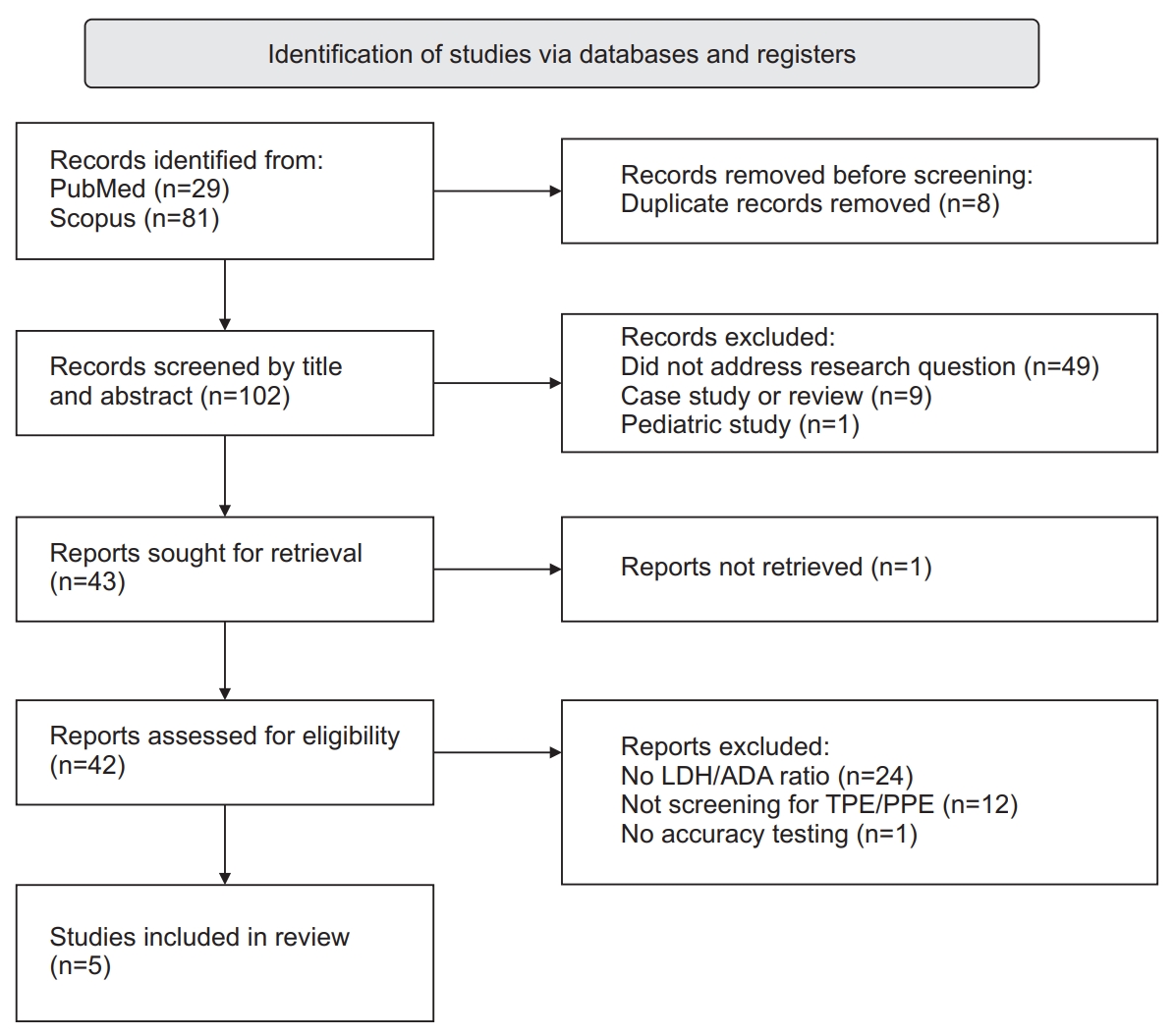
Figure┬Ā1.
Table┬Ā1.
| Study | Country | Design and Incidence of TB | Sample size | Inclusion criteria | Exclusion criteria | Classification of effusion | Criteria for TPE | Criteria for PPE |
|---|---|---|---|---|---|---|---|---|
| Beukes et al. (2021) [6] | South Africa | Prospective, TB incidence: 500/100,000 | 228 | (1) Confirmed TPE | (1) Undiagnosed exudates | (1) TPE | (1) Microbiological or histologic confirmation of TPE | Not stated |
| (2) Confirmed chronic nonspecific pleuritis | (2) Chronic nonspecific pleuritis | |||||||
| (2) Missing ADA/LDH data | (3) MPE | |||||||
| (3) Confirmed MPE | (4) PPE | |||||||
| (4) Confirmed PPE | (5) Miscellaneous | |||||||
| Blakiston et al. (2018) [11] | New Zealand | Retrospective, TB incidence: 6.4/100,000 | 1,637 | (1) Age at least 15 years | (1) Duplicate samples | (1) TPE | (1) Positive culture | (1) Documented clinical diagnosis |
| (2) Exudative effusion or unknown nature | (2) Non-TPE | (2) Absence of an alternative cause | ||||||
| (3) Transudative effusion | ||||||||
| Ho et al. (2022) [12] | Taiwan | Retrospective, TB incidence: 43.9/100,000 [13] | 311 | (1) First-time thoracocentesis for undiagnosed effusion | (1) Incomplete data | (1) TPE | (1) Positive sputum, pleural, or tissue results | (1) Preceding pneumonia, bronchiectasis, lung abscess, and positive pleural sputum culture |
| (2) MPE | ||||||||
| (3) PPE | (2) Clinical response to antituberculous therapy | |||||||
| (2) ADA >40 | (4) Miscellaneous | |||||||
| Lin et al. (2021) [4] | China | Retrospective, TB incidence: 21.7/100,000 [14] | 112 | (1) Exudative effusion | Not stated | (1) TPE | (1) Microbiological or histologic confirmation of TPE | (1) Bacterial pneumonia, lung abscess, bronchiectasis, with no evidence of TPE |
| (2) MPE | ||||||||
| (3) UPPE | ||||||||
| (4) CPPE | (2) Clinical response to antituberculous therapy | |||||||
| (5) CTD-effusion | ||||||||
| Wang et al. (2017) [5] | China | Retrospective, TB incidence: 103.5/100,000 [15] | 119 | (1) Confirmed TPE | Not stated | (1) TPE | (1) Histological confirmation | (1) Exudative effusions associated with bacterial pneumonia, lung abscesses, etc. |
| (2) Confirmed MPE | (2) PPE | (2) Clinical response to antituberculous therapy | (2) Absence of MTB in pleural fluid | |||||
| (3) Absence of TB histologic features | ||||||||
| (3) No/minimal pleural effusion in the last 12 months | (4) Remission and recovery for at least 3 months at follow-up |
TB: tuberculosis; TPE: tuberculous pleural effusion; PPE: parapneumonic effusion; MPE: malignant pleural effusion; ADA: adenosine deaminase; LDH: lactate dehydrogenase; UPPE: uncomplicated parapneumonic effusion; CPPE: complicated parapneumonic effusion; CTD: connective tissue disease; MTB: Mycobacterium tuberculosis.
Table┬Ā2.
| Study | Cutoff values of LDH/ADA ratio for TPE | Sensitivity, % | Specificity, % | LR+ | LR- | PPV, % | NPV, % |
|---|---|---|---|---|---|---|---|
| Beukes et al. (2021) [6] | <25 | 97 | 62 | 2.6 | 0.1 | 86 | 89 |
| <16.2 | 91 | 76 | 3.8 | 0.1 | 90 | 79 | |
| <15 | 91 | 81 | 4.8 | 0.1 | 92 | 79 | |
| <12.5 | 86 | 88 | 7.2 | 0.2 | 94 | 72 | |
| <10 | 78 | 90 | 7.8 | 0.2 | 95 | 64 | |
| <7.5 | 64 | 96 | 16 | 0.4 | 97 | 53 | |
| Blakiston et al. (2018) [11] | <25 | 100.0 | 61.6 | 2.6 | 0 | 8.5 | 100.0 |
| <15 | 89.1 | 85 | 5.9 | 0.1 | 17.3 | 99.5 | |
| <15 and ADA>30 | 85.5 | 97.8 | 38.9 | 0.2 | 57.3 | 99.5 | |
| <25 and ADA>30 | 92.7 | 96.6 | 27.3 | 0.1 | 49 | 99.7 | |
| <15 and ADA>15 | 89.1 | 92.7 | 12.2 | 0.1 | 30.3 | 99.6 | |
| <25 and ADA>15 | 100 | 86.9 | 7.6 | 0 | 21.2 | 100 | |
| Ho et al. (2022) [12] | <14.2 | 90.4 | 74.2 | 3.5 | 0.1 | 70.2 | 92 |
| >14.5 for PPE | 79.9 | 78.5 | 3.7 | 3.7 | 75 | 82.8 | |
| Lin et al. (2021) [4] | For ADA >19.65, serum albumin >23.95, ratio <29.61 for TPE | 100.0 | 98.7 | 76.9 | 0.0 | 97.1 | 100.0 |
| Wang et al. (2017) [5] | <16.2 | 93.6 | 93.1 | 13.5 | 0.5 | NA | NA |
Table┬Ā3.
| Study |
Pleural fluid LDH, U/L |
Pleural fluid ADA, U/L |
LDH/ADA ratio |
|||
|---|---|---|---|---|---|---|
| TPE | PPE | TPE | PPE | TPE | PPE | |
| Beukes et al. (2021) [6] | 476 (314.5-781.5) | 7,782 (3,830.5-13,516.5) | 88.4 (62.8-115.5) | 160.4 (73.1-196.7) | 6.2 (3.7-9.6) | 49.3 (29.9-77.3) |
| Blakiston et al. (2018) [11] | 453 (342-647) | CPPE: 1,438 (816.5-2,960) | 58.1 (45.1-74.8) | CPPE: 31.9 (21.7-57.6) | 8.2 (5.9-11.) | CPPE: 45.8 (32.8-61.6) |
| UPPE: 260 (156-478) | UPPE: 11.1 (6.9-16.7) | UPPE: 25.7 (17.3-36.6) | ||||
| Ho et al. (2022) [12],* | 348 (223-709) | 2,271 (1,084.5-5,039.5) | 66 (53-90) | 63 (47-105) | 5.12 (3.6-9.14) | 40.77 (17.63-65.82) |
| Lin et al. (2021) [4] | 532 (144-1,783) | CPPE: 1,607 (731-10,613) | 48 (20.6-81.5) | CPPE: 32.9 (12-115.4) | 12.27 (4.48-29.34) | CPPE: 62.97 (29.87-133.92) |
| UPPE: 277 (68-1,169) | UPPE: 8.9 (1.4-19.5) | UPPE: 32.92 (12.14-101.57) | ||||
| Wang et al. (2017) [5] | 364.5 (55-1,154) | 4037 (103-48,730) | 33.5 (4.5-75.9) | 43.3 (2.0-344.1) | 10.88 (3.65-21.81) | 66.91 (9.04-411.4) |
REFERENCES
- TOOLS
-
METRICS

- ORCID iDs
-
Larry Ellee Nyanti

https://orcid.org/0000-0002-5790-3919 - Related articles


 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Data Sharing Statement
Data Sharing Statement Full text via DOI
Full text via DOI Supplement
Supplement Print
Print Download Citation
Download Citation



